Written By: Dewanshee Ingale (B.Pharm)

Yutrepia is an FDA-approved, inhaled dry-powder dosage form of treprostinil for treating pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD). This approval provides patients with a novel, practical, and efficient therapy alternative, marking a substantial development in caring for these complicated conditions. Even though treprostinil has been in practice since 2002 in various dosage forms, recently treprostinil (Yutrepia) was approved by the FDA on May 23, 2025, especially as an inhalation powder formulation for the management and control of pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD). Earlier, other inhaled forms, most remarkably an inhalation solution of treprostinil, were sanctioned in 2009 for the same indications (Tyvaso). The fundamental difference is that Yutrepia introduced the first FDA-approved dry powder inhalation dosage form of treprostinil, which proposes an entirely novel delivery technique and is based on Liquidia’s proprietary PRINT™ technology, which yields uniform, free-flowing particles intended for enhanced deep lung delivery via an easy-to-use, low-effort device requiring less inspiratory effort as compared to the prior nebulized solution.
Present data and background
Pulmonary arterial hypertension (PAH) and pulmonary hypertension with interstitial lung disease (PH-ILD) are progressive conditions that increase pulmonary artery pressure. This can be followed by right heart failure (associated with the right ventricle) and exercise intolerance. PAH was initially treated with four main mechanisms: endothelin-1, nitric oxide, prostacyclin, and bone morphogenetic protein/activin signaling, more recently. In recent times, physicians increasingly prescribe combination therapy, which is more effective for symptoms and outcomes compared to single treatment.
People with fibrotic lung diseases often develop a serious complication called pulmonary hypertension (PH-ILD), which makes their condition worse and increases the risk of death. Until recently, there were no approved treatments for this. Now, fast and effective treatment is important to manage the disease and improve outcomes. Initially the agents were unsuccessful and not safe, which revealed a lack of therapy and the need for effective and nontoxic treatment. The advent of inhaled treprostinil was innovative. The recent trials enhanced exercise capacity and reduced the severity of disease in patients with PH-ILD, which led to approval of the drug. As the traditional nebulized form was clumsy and time taking, led to decreased patient compliance. The dry inhalational powder of treprostinil was developed to provide a suitable, handy delivery system. The novel delivery technology is an enhancement in the therapy of PAH and PH-ILD, improving patient convenience and compliance in treatment.
Yutrepia: a novel approach
Treprostinil is a prostacyclin (PGI₂) analogue primarily used to treat Pulmonary Arterial Hypertension (PAH). It mimics the action of endogenous prostacyclin, a potent vasodilator and inhibitor of platelet aggregation. Treprostinil binds to IP receptors (prostacyclin receptors) on vascular smooth muscle cells. This activates adenylate cyclase, increasing cyclic AMP (cAMP) levels, which leads to smooth muscle relaxation and vasodilation, particularly in the pulmonary and systemic circulation. Treprostinil also inhibits platelet aggregation through the same cAMP-mediated pathway, which helps reduce the risk of thrombosis, a big alarm in PAH. Elevated cAMP levels also exert anti-proliferative effects on vascular smooth muscle cells, helping to prevent vascular remodelling, a characteristic of PAH progression. By mimicking prostacyclin, treprostinil can enhance endothelial cell function and reduce oxidative stress and inflammation in pulmonary arteries.
About Yutrepia and PRINT technology
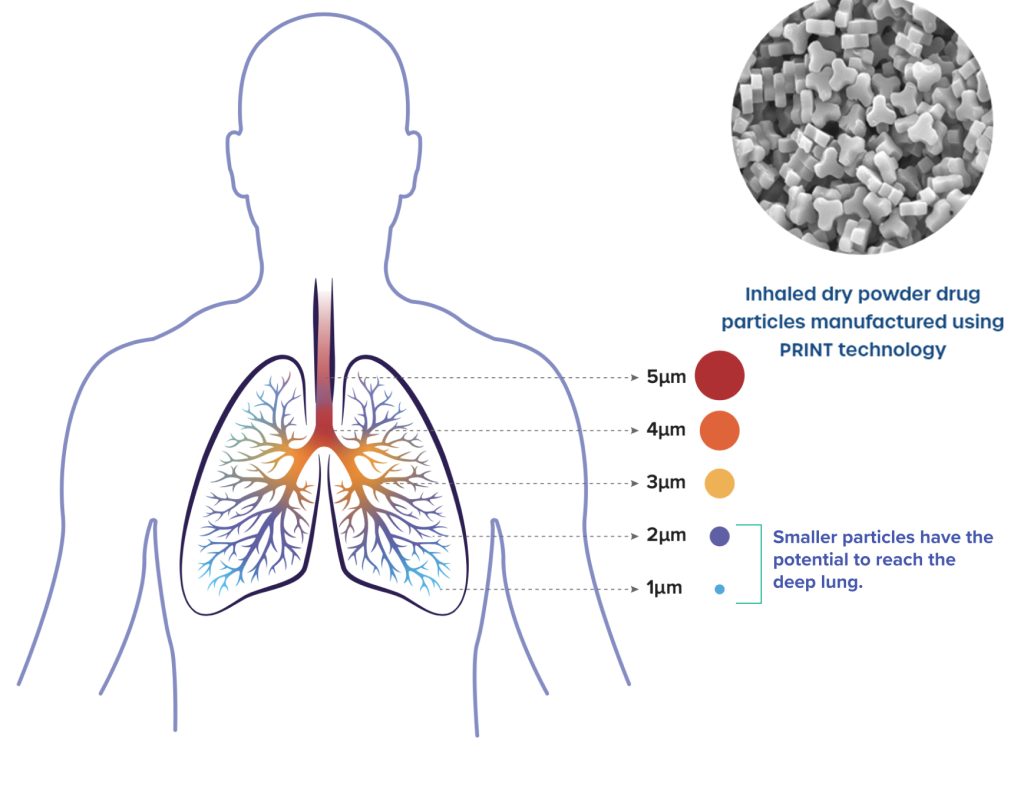
Yutrepia is developed meticulously using Liquidia’s PRINT technology. The new drug is administered via a tiny, compact device that is lightweight and could be placed in the palms of hands. Development of Yutrepia efficiently utilizes Liquidia’s PRINT technology to formulate drug particles that are precise as well as uniform in size, shape, and composition in such a way that they can deliver more in the lungs when inhaled. The particle’s diameter is found to be 1.3 μm, which implies that the size of the particles is well controlled. The particles have a three-leaf clover shape, which facilitates their ability to effectively deliver drug.
Clinical trials and approval
The sanction of Yutrepia (Treprostinil) dry inhalational powder for pulmonary arterial hypertension(PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD) was supported on the pivotal, open-label, multicentre Phase 3 INSPIRE trial (ClinicalTrials.gov Identifier: NCT03399604). Furthermore, the ongoing ASCENT study (ClinicalTrials.gov Identifier: NCT06129240) is estimating the long-term safety criteria and acceptability of Yutrepia in patients with PH-ILD.
NCT03399604 an open-label, multicentre INPSPIRE trial phase III study was designed to assess the safety and tolerability of Yutrepia (dry-powder inhalational formulation of treprostinil) in adults with PAH. Overall 121 patients aged 18 years or above were registered, including the ones who transformed from nebulized treprostinil and prostacyclin naive patients which receive up to two non-prostacyclin oral therapies. Transition patients were started with Yutrepia at a dose that is equivalent to the prior nebulized dosing. The prostacyclin-naive patients were treated with 26.5 mcg four times daily, with dose modification in 26.5mcg in which growth can be allowed for both the groups.
The preliminary aims were to evaluate the occurrence of adverse events (AEs) and serious adverse (SAEs) throughout the entire study. Investigative efficacy parameters including changes in the 6-minute walk distances, NYHA (New York heart association), NT-proBNP levels (N-terminal pro-B-type natriuretic peptide) exposed that most patients stayed stable or enhanced over the one-year treatment time lapse. Moreover, quality of life scores enhanced, it was being observed that most of the patients preferred the Yutrepia inhaler over earlier used nebulized devices. In general, Yutrepia was discovered to be a suitable and very well-tolerated inhaled prostacyclin treatment therapy for PAH patients, assisting its use as a novel therapy option in this population.
The primary endpoints were the occurrence of AEs and SAEs. Throughout the one-year treatment period, 80% of the transition group and 96% of the prostacyclin-naive group modified to a dose of not less than 79.5 mcg four times daily, with at least one patient reaching 212 mcg daily four times. Majority of adverse effects were found to be mild to modest and steady prostacyclin therapy, comprising cough, headache, upper respiratory infection, dyspnoea, and throat irritation. Most of the patients remained stable or improved during the study.
Clinical trial NCT06129240, known as the ASCENT study, is an ongoing Phase 3, open-label, multicenter trial. It is designed to evaluate the long-term safety and tolerability of Yutrepia, a dry powder inhaled formulation of treprostinil, in patients with pulmonary hypertension (PH) and PH associated with interstitial lung disease (PH-ILD). The trial is still ongoing and recruiting participants.
Safety profile
Yutrepia (treprostinil) inhalation powder has established an appropriate safety and tolerability outline n clinical trials, remarkably in the pivotal phase III INSPIRE study. Approximately all the patients (99.2%) observed at least one adverse event (AE), while most of these being mild (47.9%) or moderate (28.1%) in seriousness. Harmful AEs were unusual (3.3%). In this clinical trials there were no serious adverse effects or mortality reported throughout the study.
The frequently reported adverse reactions <10% included not so serious side effects like cough, headache, and upper respiratory tract infections. The side effects were consistent with the known safety profile of the inhalational powder therapies and did not prevent patients from continuing treatment.
The patients who received higher percentage of prostacyclin-naive observed dose related AEs as compared the ones who were transitioned from the nebulized treprostinil (84.8% vs 72.7%). Although in general the incidence of moderate or severe AEs were quite controllable. Approximately 12.4% of patients did not continue the treatment due to AEs, among which 9.1% of these events were related to Yutrepia.
The medical monitor significantly did not observe any of the SAEs related to the Yutrepia in the trials. The maximum number of hospitalizations was because of unrelated causes like accidents, comorbidities, or any kind of viral infection, for example, COVID-19. There were no deaths reported during the study of the drug.
Impact and future viewpoint
The FDA approval of Yutrepia (treprostinil) provides with a new delivery system of inhalation dosage form by using dry inhalation powder for the treatment of pulmonary arterial hypertension and pulmonary hypertension associated interstitial lung disease this led to enhanced exercise capability, suitability, and quality of life of patients who have limited treatment alternatives. The importance of constant research and invention in cardiopulmonary diseases were highlighted by the approval. Directing to extend therapeutic pathways and improve patient results.
The upcoming research will discover the longstanding profits, ideal dosing approaches and the ability of Yutrepia to be utilized in wider patient populations. The present and upcoming studies, like the ASCENT trials, will moreover provide extra data on long-term safety and efficacy. With Yutrepia being more widely available, its distinctive dry powder dosage form and simple inhaler are probably to expand patient acceptance to prostacyclin therapy, leading to improved disease treatment for patients with PAH and PH-ILD.
Refrences
YUTREPIA is an FDA-approved, inhaled dry-powder formulation of treprostinil indicated for the treatment of pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD) https://liquidia.com/pipeline-and-products
FDA approval history for Yutrepia (treprostinil) used to treat Pulmonary Arterial Hypertension; Pulmonary Hypertension Associated with Interstitial lung disease (PH-ILD)https://www.drugs.com/history/Yutrepia.html
U.S. FDA Approves Liquidia’s YUTREPIA™ (treprostinil) Inhalation Powder for Patients with Pulmonary Arterial Hypertension (PAH) and Pulmonary Hypertension Associated with Interstitial Lung Disease (PH-ILD)https://liquidia.com/news-releases/news-release-details/us-fda-approves-liquidias-Yutrepiatm-treprostinil-inhalation
FDA approval history for Tyvaso (treprostinil) used to treat Pulmonary Arterial Hypertension. Supplied by United Therapeutics Corporation https://www.drugs.com/history/tyvaso.html
Pulmonary Hypertension in Interstitial Lung Disease: Management Options to Move beyond Supportive Care https://pmc.ncbi.nlm.nih.gov/articles/PMC10200699/
Therapeutic Potential of Treprostinil Inhalation Powder for Patients with Pulmonary Arterial Hypertension: Evidence to Date https://pmc.ncbi.nlm.nih.gov/articles/PMC11162632/
INSPIRE: Safety and tolerability of inhaled Yutrepia (treprostinil) in pulmonary arterial hypertension (PAH) https://pmc.ncbi.nlm.nih.gov/articles/PMC9400582/
Liquidia Announces the Publication of Long-Term Clinical Data from Completed INSPIRE Study in the Journal Pulmonary Circulation https://liquidia.com/news-releases/news-release-details/liquidia-announces-publication-long-term-clinical-data-completed
FDA Approves Yutrepia (treprostinil) Inhalation Powder for Pulmonary Arterial Hypertension (PAH) and Pulmonary Hypertension Associated with Interstitial Lung Disease (PH-ILD) https://www.drugs.com/newdrugs/fda-approves-Yutrepia-treprostinil-inhalation-powder-pulmonary-arterial-hypertension-pah-pulmonary-6529.html
Transitioning from Parenteral Treprostinil to LIQ861 in a Patient with PAH San Francisco, CA https://liquidia.com/publications
Hill, N.S. et al., INSPIRE: A Phase 3 Open-Label, Multicenter Study to Evaluate the Safety and Tolerability of LIQ861 in Pulmonary Arterial Hypertension (PAH) (Investigation of the Safety and Pharmacology of Dry Powder Inhalation of Treprostinil NCT03399604), The Journal of Heart and Lung Transplantation, Volume 38, Issue 4, S11
Hill NS, Feldman JP, Sahay S, INSPIRE study investigators. INSPIRE: Safety and tolerability of inhaled Y et al, utrepia (treprostinil) in pulmonary arterial hypertension (PAH). Pulm Circ. 2022 Jul 1;12(3):e12119. doi: 10.1002/pul2.12119. PMID: 36034402; PMCID: PMC9400582.
Roscigno R, Vaughn T, Anderson S, Wargin W, Hunt T, Hill NS. Pharmacokinetics and tolerability of LIQ861, a novel dry-powder formulation of treprostinil. Pulm Circ. 2020 Nov 19; 10(4):2045894020971509. Doi: 10.1177/2045894020971509. PMID: 33282202; PMCID: PMC7682229.
An Open-Label ProSpective MultiCENTer Study to Evaluate Safety and Tolerability of Dry Powder Inhaled Treprostinil in PH (ASCENT), ClinicalTrials.gov ID NCT06129240, https://clinicaltrials.gov/study/NCT06129240
The article is extensively reviewed and fact-checked by the editorial team team of pharmacally.com

