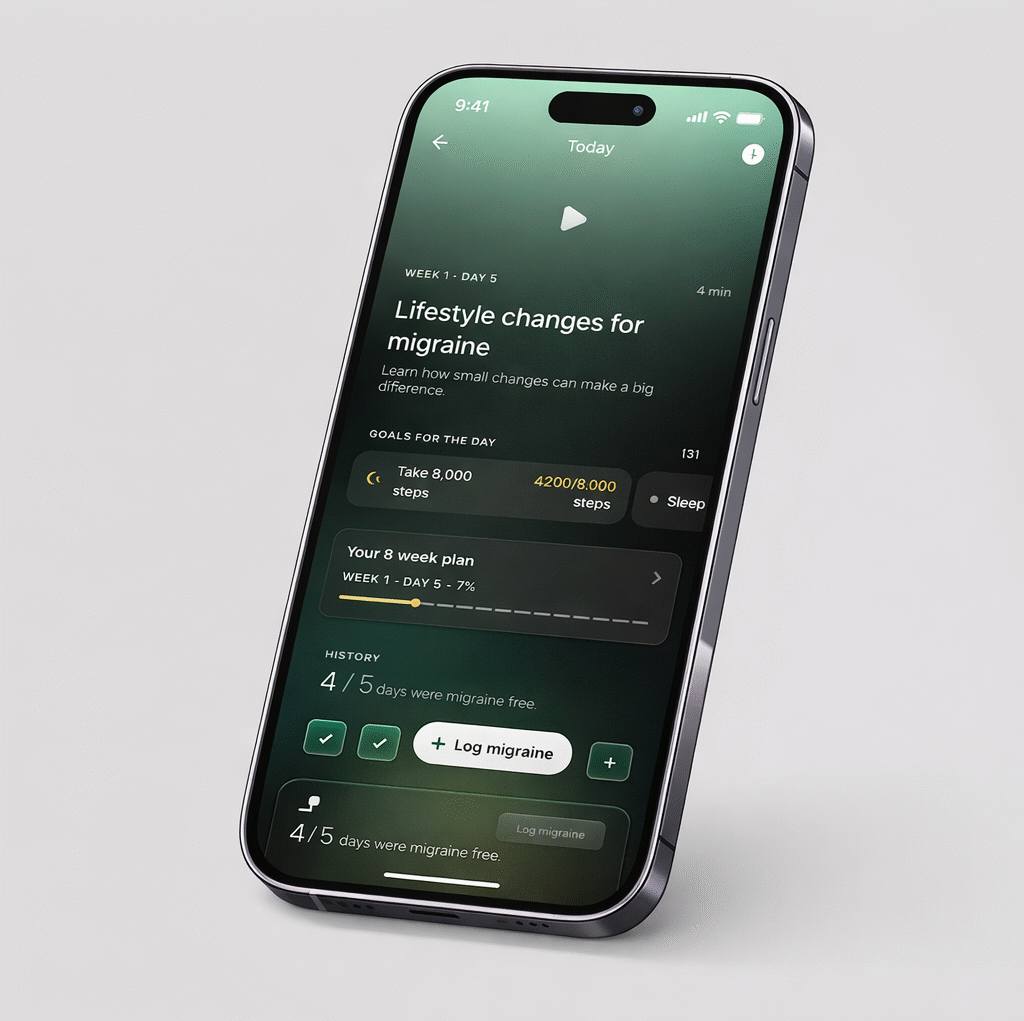
Ultrahuman partners with Click Therapeutics to launch Migraine PowerPlug, a digital therapeutic tool combining wearable biometrics and FDA-authorized technology to provide personalized migraine insights and guided care.
Written By: Sana Khan BPharm
Reviewed By: Pharmacally Editorial Team
Ultrahuman, a global company in wearable health technology, has joined hands with Click Therapeutics to create the Migraine PowerPlug, a digital tool designed to help people better manage migraine. The feature will be integrated into the Ultrahuman platform and will draw on Click’s FDA-authorized prescription digital therapeutic, CT-132.
The companies aim to provide personalized, real-time guidance to the more than one billion people worldwide who live with migraine. Current estimates show that 15 to 20 percent of the global population experiences migraine, and women are affected almost three times more often than men.
Addressing a Major Women’s Health Need
Migraine remains a chronic neurological disorder with no cure and stands as the leading cause of disability among women aged 15 to 49. The condition disrupts work, family responsibilities, and overall quality of life. Ultrahuman has placed women’s health at the center of its product strategy, especially after acquiring viO and its OvuSense technology.
Hormonal changes play a key role in migraine patterns for many women. Ultrahuman believes that continuous wellness data can help users understand how these fluctuations relate to migraine frequency and severity, enabling earlier and more informed action.
Care Beyond the Clinic
Most migraine care still depends on occasional doctor visits, while triggers and symptoms occur daily. Migraine PowerPlug seeks to close this gap by combining wearable biomarker data with evidence-based digital therapy. CT-132, the technology behind the program, is the first FDA-authorized digital treatment for preventive migraine care and is intended to be used alongside standard medication.
Unlike basic tracking apps, the new feature will apply therapeutic principles to help users link biometric changes with potential triggers and respond through guided behavioral strategies.
Focus on Sleep, Stress, and Recovery
The tool will monitor trends in sleep quality, heart rate variability, stress load, and physical activity. It will then convert these signals into practical lifestyle recommendations, including goals for movement, rest, and hydration tailored to each user’s migraine history.
Many patients already notice that poor sleep or high stress worsens their attacks. The platform aims to make these connections clearer and help users build daily habits that reduce migraine burden over time.
Mohit Kumar, CEO of Ultrahuman, said the collaboration will allow users to move from simple tracking to real action. He explained that the PowerPlug will help people understand migraine-related patterns in real time and turn those insights into meaningful guidance.
David Benshoof Klein, CEO and founder of Click Therapeutics, said the partnership brings a clinically validated solution to the growing wearables market. He noted that combining digital treatment with consumer biometrics can address an important gap in over-the-counter migraine care.
Launch Timeline
Ultrahuman plans to release Migraine PowerPlug in early 2026 after completing a pilot phase. The rollout will cover the United States, Canada, the European Union, India, Australia, and additional regions.
Reference
Ultrahuman and Click Therapeutics partner to launch the world’s first biomarker-driven migraine management tool based on FDA-authorized technology, 14 January 2026, https://www.clicktherapeutics.com/news/ultrahuman-and-click-therapeutics-partner-to-launch-the-worlds-first-biomarker-driven-migraine-management-tool-based-on-fda-authorized-technology
Ultrahuman and Click Therapeutics partner to launch the world’s first biomarker-driven migraine management tool based on FDA-authorized technology, 14 January 2026, https://cyborg.ultrahuman.com/press-releases/ultrahuman-click-therapeutics-migraine-powerplug