Written By: Pharmacally Medical News Desk
Article updated on 17 December 2025 for the Addition of FDA Approval Status.
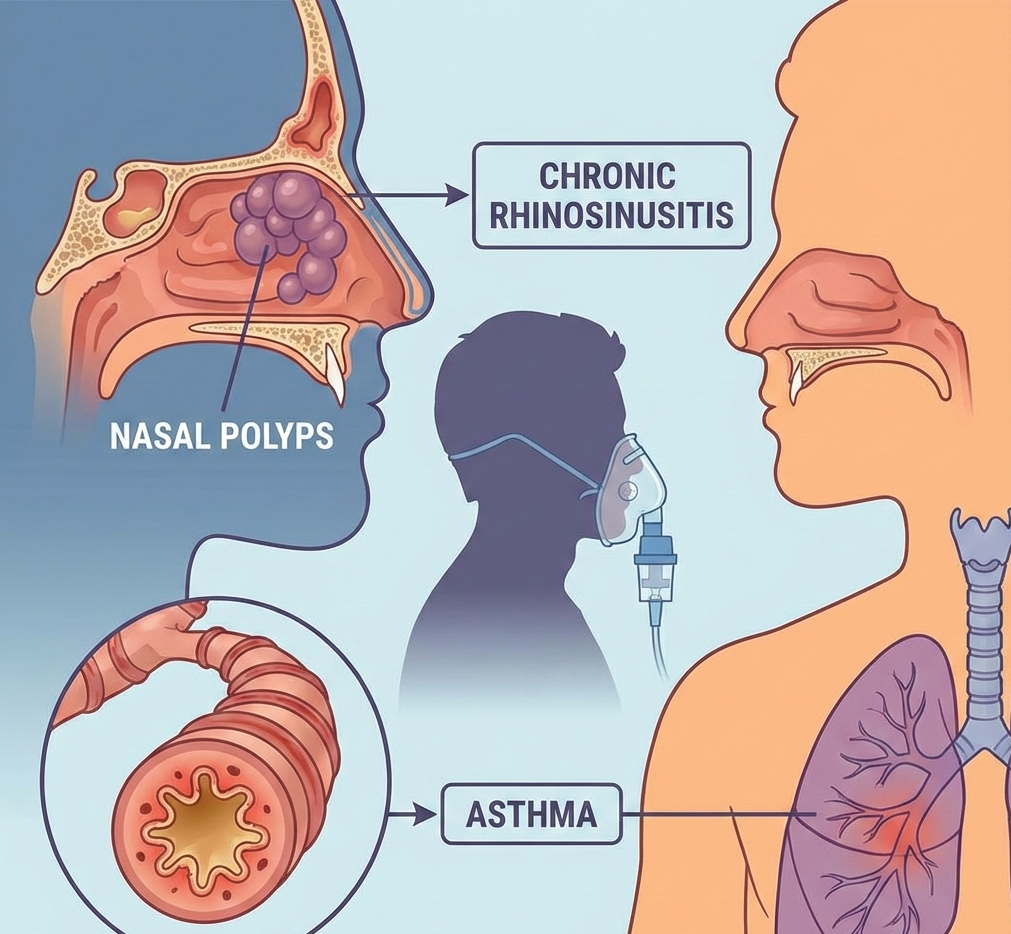
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorization to GSK for Exdensur (depemokimab), a biologic therapy approved for the treatment of asthma driven by type 2 inflammation and for chronic rhinosinusitis with nasal polyps (CRSwNP) in adults and adolescents. This approval marks the first global authorization for depemokimab and introduces a twice-yearly treatment option for patients whose disease remains inadequately controlled despite existing therapies.
About Exdensur
Exdensur (depemokimab) is an ultra-long-acting monoclonal antibody designed to target interleukin-5 (IL-5), a signalling protein that plays a central role in type 2 inflammation. IL-5 drives the growth and activation of eosinophils, a type of white blood cell often elevated in people with severe asthma and CRSwNP. By binding IL-5 and preventing it from activating its receptor, depemokimab reduces eosinophil-mediated inflammation in the airways and sinus tissues.
The extended half-life of depemokimab allows for subcutaneous dosing just once every six months, a significant reduction in treatment burden compared with many existing biologics that require more frequent injections.
Approved Indications in the UK
Under the MHRA approval, Exdensur can be used in the following Indications:
Severe Asthma with Type 2 Inflammation: As an add-on maintenance therapy for patients aged 12 years and older whose asthma remains inadequately controlled on medium- or high-dose inhaled corticosteroids plus at least one other controller medication.
Chronic Rhinosinusitis with Nasal Polyps (CRSwNP): As an add-on to intranasal corticosteroids in adults with severe CRSwNP that remains poorly controlled despite systemic corticosteroid treatment and/or surgery.
These indications reflect a focus on type 2 inflammatory pathways, which underlie both conditions and are particularly responsive to targeted biologic therapy.
Clinical Evidence Supporting Approval
The MHRA decision is based on data from pivotal phase III programs:
In patients with eosinophilic asthma, depemokimab significantly reduced the annualized rate of clinically significant asthma exacerbations compared with placebo when added to standard of care. For the SWIFT-1 and SWIFT-2 phase III trials in severe eosinophilic asthma, twice-yearly depemokimab reduced the annualized rate of clinically significant exacerbations by 58% in SWIFT-1 and 48% in SWIFT-2 over 52 weeks, more than halving asthma attacks in patients with severe asthma driven by type 2 inflammation.
In the phase III ANCHOR trials, depemokimab demonstrated significant improvements in nasal polyp size scores and nasal obstruction symptoms compared with placebo, confirming its efficacy as an add-on treatment in adults with severe chronic rhinosinusitis with nasal polyps.
Across trials, depemokimab was generally well tolerated, with side effect rates similar to placebo groups.
Administration and Dosing
Exdensur is given as a pre-filled injection under the skin once every six months. This dosing schedule aims to improve adherence and reduce clinic visits for patients requiring biologic therapy.
Safety and Monitoring
As with all biologic therapies, monitoring for adverse effects and long-term safety remains important. Common reactions with similar agents include injection site reactions, headache, and fatigue. The MHRA will continue to monitor safety as Exdensur enters clinical use.
Depemokimab Development Programme
Beyond its approved indications, depemokimab is being evaluated in several ongoing phase III clinical programs targeting other diseases driven by type 2 inflammation. These include the OCEAN study in eosinophilic granulomatosis with polyangiitis (EGPA) and the DESTINY trial in hyper-eosinophilic syndrome (HES). In addition, GSK has initiated the ENDURA-1, ENDURA-2, and VIGILANT phase III trials to assess depemokimab as an add-on therapy in patients with uncontrolled moderate to severe COPD with type 2 inflammation.
Regulatory Landscape and Next Steps
The UK approval is the first global nod for depemokimab. A positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) suggests a likely EU approval in early 2026, and following approvals in the UK and the United States, regulatory decisions in Japan, the European Union, and China are expected through the first half of 2026.
Kaivan Khavandi, Senior Vice President and Global Head of Respiratory, Immunology and Inflammation R&D at GSK, said the UK approval of Exdensur, the first globally, marks an important advance in respiratory care. He noted that the ultra-long-acting biologic offers sustained efficacy with just two doses a year, helping reduce asthma exacerbations, hospitalizations, and the risk of long-term lung damage.
Julian Beach, Interim Executive Director for Healthcare Quality and Access at the MHRA, said that asthma and CRSwNP affect a large number of people in the UK and can remain difficult to manage despite available treatments. He noted that the approval of depemokimab offers an additional option for patients whose symptoms are not adequately controlled and added that the MHRA will continue to closely monitor the medicine’s safety and effectiveness.
Exdensur (depemokimab) represents a new treatment option for people with severe asthma driven by type 2 inflammation and for adults with difficult-to-control CRSwNP. Its twice-yearly dosing sets it apart from many existing biologics and may make it a valuable addition to personalized respiratory care in the UK and potentially beyond.
Update on 17 December 2025
Following its first global approval in the UK, Exdensur (depemokimab) has now also been approved by the US Food and Drug Administration (FDA) for the treatment of asthma with type 2 inflammation and chronic rhinosinusitis with nasal polyps. The FDA decision further validates the clinical evidence supporting depemokimab’s efficacy and safety and marks a significant regulatory milestone for GSK, expanding patient access to a twice-yearly biologic treatment option in one of the world’s largest respiratory care markets.
References
Exdensur (depemokimab) approved in the UK for treatment of asthma with type 2 inflammation and chronic rhinosinusitis with nasal polyps, 15 December 2025, https://www.gsk.com/en-gb/media/press-releases/exdensur-depemokimab-approved-in-the-uk-for-treatment-of-asthma-with-type-2-inflammation-and-chronic-rhinosinusitis-with-nasal-polyps/
UK approves the first twice-yearly biological medicine for asthma and severe chronic rhinosinusitis with nasal polyps, 15 December 2025, MHRA, https://www.gov.uk/government/news/uk-approves-the-first-twice-yearly-biological-medicine-for-asthma-and-severe-chronic-rhinosinusitis-with-nasal-polyps
David J. Jackson et al, Twice-Yearly Depemokimab in Severe Asthma with an Eosinophilic Phenotype, N Engl J Med 2024;391:2337-2349, DOI: 10.1056/NEJMoa2406673
Gevaert, Philippe et al, Efficacy and safety of twice per year depemokimab in chronic rhinosinusitis with nasal polyps (ANCHOR-1 and ANCHOR-2): phase 3, randomised, double-blind, parallel trials, The Lancet, Volume 405, Issue 10482, 911 – 926, DOI:10.1016/S0140-6736(25)00197-7