Written By: Pharmacally Medical News Desk
Takeda has announced positive landmark Phase 3 results for zasocitinib (TAK-279), an investigational, once-daily oral therapy for adults with moderate-to-severe plaque psoriasis. The company reported that zasocitinib met all primary and key secondary endpoints across two pivotal Phase 3 studies, demonstrating rapid, deep, and sustained skin clearance. In the topline results shared in the announcement, more than half of treated patients achieved near-complete skin clearance, while around one-third reached complete clearance by week 16. These findings signal the potential of zasocitinib to redefine expectations for oral psoriasis therapies.
Commenting on the announcement, Christophe Weber, president and chief executive officer of Takeda, highlighted the significance of the results, describing them as a major step forward for patients living with plaque psoriasis who are seeking effective and convenient treatment options. He emphasized Takeda’s commitment to translating advanced science into meaningful patient outcomes and underscored the importance of expanding therapeutic choices beyond injectable biologics.
Zasocitinib is a selective allosteric tyrosine kinase 2 (TYK2) inhibitor designed to modulate key inflammatory pathways involved in psoriasis. TYK2 is an intracellular enzyme that plays a central role in signalling pathways driven by cytokines such as interleukin-23, interleukin-12, and type I interferons, all of which are known contributors to psoriatic inflammation. By selectively inhibiting TYK2 without directly blocking closely related JAK enzymes, zasocitinib aims to deliver strong anti-inflammatory effects while minimizing the safety concerns historically associated with broader JAK inhibition. The convenience of once-daily oral dosing (25 mg/50 mg) further positions zasocitinib as a potentially attractive alternative for patients who prefer pills over injections.
LATITUDE Psoriasis Phase 3 program
The Phase 3 LATITUDE Psoriasis program consists of two global, randomized, double-blind, placebo- and active-controlled trials: LATITUDE-PsO-1 (NCT06088043) and LATITUDE-PsO-2 (NCT06108544). Both studies enrolled adults with moderate-to-severe plaque psoriasis and evaluated once-daily oral zasocitinib versus placebo and an active comparator. The coprimary endpoints were achievement of PASI 75 (75 percent improvement in Psoriasis Area and Severity Index) and a static Physician’s Global Assessment (sPGA) score of 0 or 1 at week 16.
Takeda reported that zasocitinib met all coprimary endpoints in both studies and demonstrated robust performance across a broad range of ranked secondary endpoints. Notably, more than 50 percent of patients achieved PASI 90, reflecting near-complete skin clearance, and approximately 30 percent achieved PASI 100, indicating complete clearance, by week 16. Clinically meaningful responses were observed early and continued to improve through later assessment points.
Zasocitinib demonstrated a favorable safety profile through week 24 in the LATITUDE Phase 3 trials, consistent with prior Phase 2b data and showing no new safety signals. The most common adverse events (AEs) were mild to moderate, including upper respiratory tract infection (URI, occurring in approximately 15-20% of patients), nasopharyngitis (10-15%), and acne (8-12%), which resolved without discontinuation in most cases.
Andy Plump, M.D., Ph.D., president of R&D at Takeda, noted that the depth of skin clearance observed with zasocitinib represents a new benchmark for oral therapies in psoriasis. He pointed out that the consistency of results across both pivotal trials and the favorable safety profile reinforces confidence in the TYK2 mechanism and validates Takeda’s strategic investment in immunology research.
Following the positive outcomes from the LATITUDE Phase 3 program, Takeda confirmed its intention to advance zasocitinib toward global regulatory submissions. The company indicated that regulatory filings are anticipated in 2026, supported by the robust efficacy and safety data generated across both pivotal studies. Takeda also plans to present additional long-term efficacy and safety analyses from the LATITUDE trials at upcoming medical congresses and submit the full datasets for peer-reviewed publication.
The zasocitinib is currently being evaluated in a head-to-head Phase 3 study against deucravacitinib in plaque psoriasis, aimed at directly comparing efficacy and safety between the two oral TYK2 inhibitors. In addition, Phase 3 studies in psoriatic arthritis are ongoing, and Phase 2 trials in Crohn’s disease and ulcerative colitis are underway.
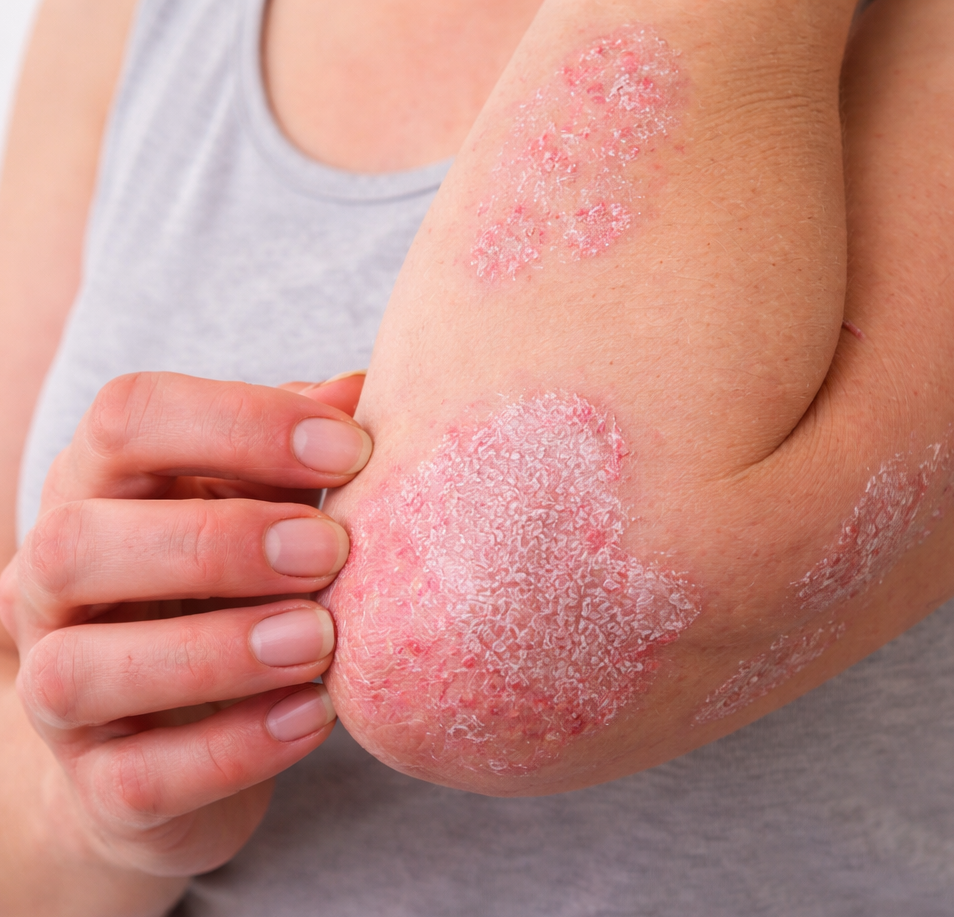
Understanding plaque psoriasis
Plaque psoriasis is a chronic, immune-mediated inflammatory skin disease characterized by raised, red plaques covered with silvery scales, most commonly affecting the elbows, knees, scalp, and lower back. The condition is not only visible but also physically and emotionally burdensome, often associated with itching, pain, and impaired quality of life. Psoriasis is a serious systemic disease, with links to comorbidities such as psoriatic arthritis, cardiovascular disease, and metabolic disorders. Globally, plaque psoriasis affects tens of millions of people, making it one of the most prevalent chronic inflammatory skin conditions.
References
Takeda’s Zasocitinib Landmark Phase 3 Plaque Psoriasis Data Show Promise to Deliver Clear Skin in a Once-Daily Pill, Catalyzing a New Era of Treatment, 18 December 2025, https://www.takeda.com/newsroom/newsreleases/2025/takeda-zasocitinib-phase-3-plaque-psoriasis-data-once-daily-pill/
A Study About How Well TAK-279 Works and Its Safety in Participants with Moderate-to-severe Plaque Psoriasis During 52 Weeks of Treatment, ClinicalTrials.gov ID NCT06088043, https://clinicaltrials.gov/study/NCT06088043
A Study About How Well TAK-279 Works and Its Safety in Participants With Moderate-to-severe Plaque Psoriasis During 60 Weeks of Treatment With a Withdrawal and Retreatment Period, ClinicalTrials.gov ID NCT06108544, https://clinicaltrials.gov/study/NCT06108544