Written By: Pharmacally Medical News Desk
The Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom has granted marketing authorization for SIILTIBCY®, a novel tuberculosis (TB) skin test developed by Serum Institute of India Pvt. Ltd. (SIIPL) and its UK subsidiary Serum Life Sciences Ltd (SLS-UK). This approval marks the first marketing authorization granted to Serum under the “Serum” name in the UK, expanding SIILTIBCY®’s availability beyond prior European Commission approval earlier in 2025.
SIILTIBCY® is indicated as a diagnostic aid for detecting Mycobacterium tuberculosis infection, including active TB disease, in adults and children aged 28 days and older. The test uses recombinant TB-specific antigens rdESAT-6 and rCFP-10 that trigger a delayed-type hypersensitivity reaction when intradermally injected, provoking an immune response visible as skin induration. This test shows high sensitivity and specificity regardless of prior BCG vaccination status, making it effective in diverse patient populations.
The MHRA approval supports Serum Life Sciences Ltd as the Marketing Authorization Holder responsible for regulatory engagement and market rollout in the UK. This regulatory milestone underscores Serum Institute of India’s growing footprint in highly regulated markets and reinforces its commitment to global health through accessible and high-quality diagnostics to support TB elimination efforts globally.
SIILTIBCY® has been rigorously evaluated in multiple clinical studies including over 2000 participants, demonstrating a favorable safety profile. The most common side effect noted was itching at the injection site. As a prescription-only diagnostic tool, it is intended for use by healthcare professionals and complements existing TB testing methodologies.
The Serum Institute of India, Pune part of Cyrus Poonawalla Group, is the world’s largest vaccine manufacturer with a strong commitment to affordable vaccines and diagnostics. Its innovative product portfolio now includes SIILTIBCY®, further enhancing diagnostic capabilities against tuberculosis in the UK and Europe, contributing to improved public health outcomes.
Mechanism of Action: How SIILTIBCY Detects Tuberculosis Infection
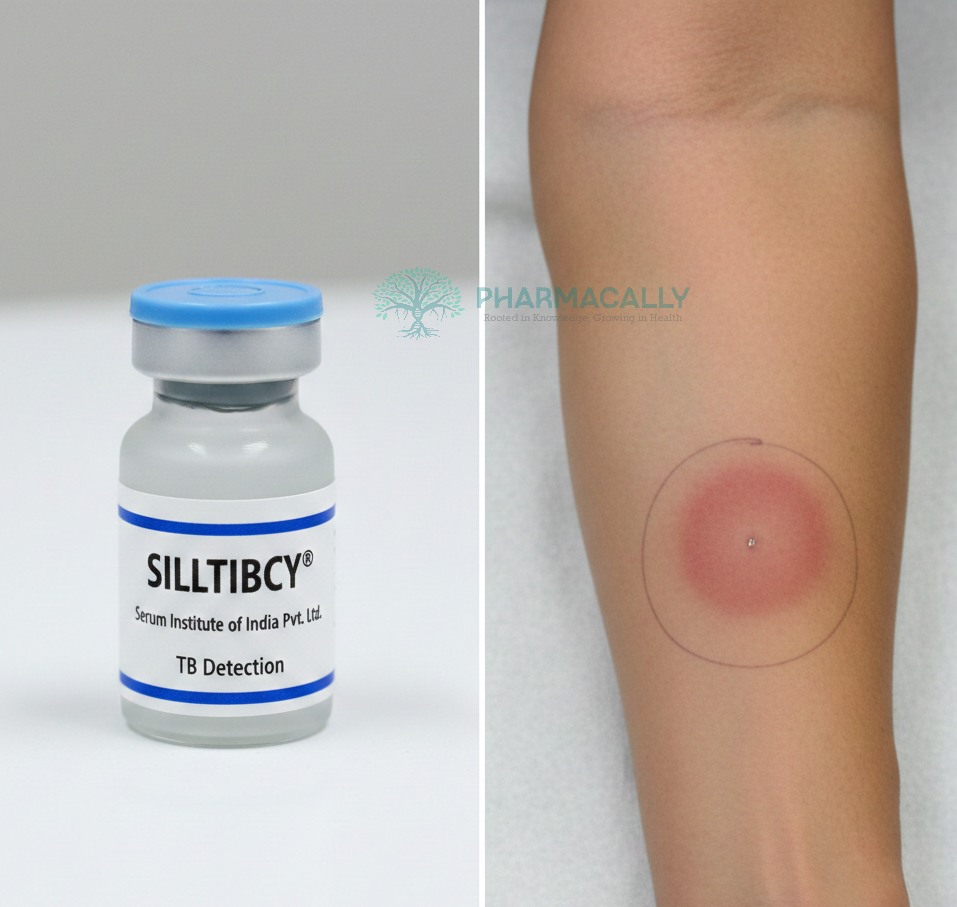
SIILTIBCY operates as a skin test that detects infection with Mycobacterium tuberculosis by eliciting a specific immune response. The test is administered as an intradermal injection on the forearm, containing two recombinant proteins called rdESAT-6 and rCFP-10, which are unique antigens specific to M. tuberculosis. When these antigens enter the skin of a person infected with tuberculosis, their immune system recognizes them and mounts a delayed-type hypersensitivity reaction.
This immune response causes localized inflammation and induration (hardening and swelling) at the site of injection, which typically develops within 48 to 72 hours. A healthcare professional measures the diameter of this induration; an induration size of 5 millimeters or more indicates a positive test, suggestive of TB infection or active disease. Unlike traditional tuberculin skin tests, SIILTIBCY offers higher specificity by avoiding false positives related to prior Bacillus Calmette-Guérin (BCG) vaccination, thereby improving diagnostic accuracy.
This mechanism allows SIILTIBCY to serve as an effective and reliable diagnostic aid for tuberculosis infection in both adults and children, supporting early detection and appropriate clinical management. The test is intended for use by trained healthcare providers in clinical settings, complementing other TB diagnostic methods with its antigen-specific sensitivity and safety profile.
For more detail, regulatory documents such as the Summary of Product Characteristics and Patient Information Leaflet will be available on the MHRA website soon. This authorization advances TB diagnostic technology and supports better targeted testing and treatment strategies in the UK healthcare system.
References
MHRA Grants Marketing Authorization for SIILTIBCY® – A Novel Tuberculosis Skin Test in the UK, Serum Institute of India, 18 November 2025, https://www.seruminstitute.com/MHRA_Grants_Marketing_Authorisation_for_SIILTIBCY%E2%80%93A_Novel_Tuberculosis_Skin_Test_in_the_UK.php
SIILTIBCY, Summary of Product Characteristics, https://www.ema.europa.eu/en/documents/product-information/siiltibcy-epar-product-information_en.pdf
MHRA approves Siiltibcy as a diagnostic aid for Mycobacterium tuberculosis infection, including disease, 06 November 2025, Medicines and Healthcare products Regulatory Agency, https://www.gov.uk/government/news/mhra-approves-siiltibcy-as-a-diagnostic-aid-for-mycobacterium-tuberculosis-infection-including-disease