Written By: Pharmacally Medical News Desk
French biopharmaceutical firm Ipsen has confirmed that its pivotal Phase II FALKON trial of fidrisertib in patients with the ultra-rare genetic bone disorder fibrodysplasia ossificans progressiva (FOP) did not achieve its primary endpoint. The update released on 19 December 2025 stated that the study will be closed following the negative outcome.
The FALKON trial (NCT05039515) was designed as the largest Phase II evaluation in FOP to date, enrolling 113 pediatric and adult participants with confirmed pathogenic variants of the disease. Ipsen had hoped that fidrisertib, an oral small molecule targeting pathogenic ALK2 kinase variants, would reduce the formation of new abnormal bone (heterotopic ossification, HO) compared with placebo. The primary endpoint measured the annualized change from baseline in HO volume, but the treatment failed to show a statistically significant reduction. As a result, the company will discontinue the study.
Christelle Huguet, PhD, EVP and Head of Research and Development at Ipsen, said the FALKON results are disappointing for patients and the FOP community but stressed that the data will still add meaningful insights to ongoing FOP research and disease management. She highlighted the scale and commitment behind the study, which enrolled 113 patients globally over more than five years, and thanked patients, caregivers, the FOP community, and clinical experts for their significant contribution to advancing understanding of this devastating disease.
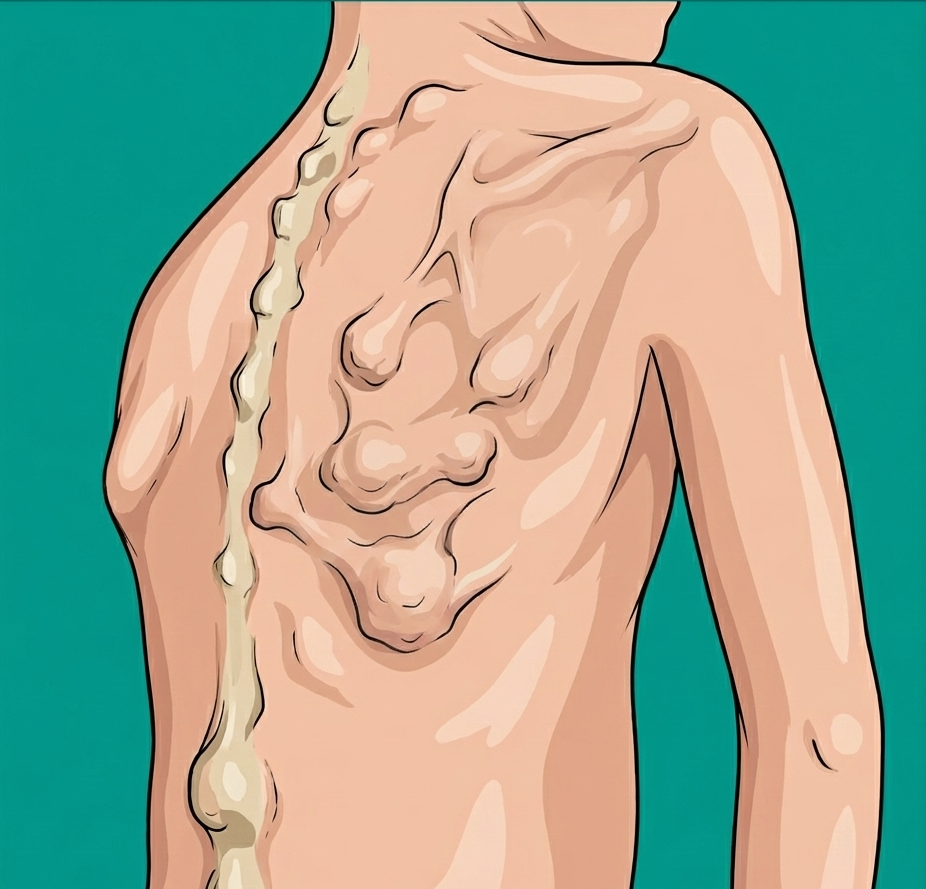
FOP is an ultra-rare connective tissue disease characterized by progressive and irreversible bone formation in muscles, tendons, and ligaments, driven by mutations in the ACVR1/ALK2 gene. The condition typically presents in early childhood, often around age five, and leads to severe functional impairment and significantly shortened life expectancy. Estimates suggest a global prevalence of about 1.36 per million individuals, with roughly 900 diagnosed patients worldwide.
Ipsen’s FALKON program included three sequential parts: a global placebo-controlled Phase II segment (Part A), followed by a crossover or continuation phase for patients initially on placebo (Part B), and a long-term extension for those showing response (Part C). The study spanned multiple years and was among the most ambitious clinical efforts in this indication.
Ipsen had previously secured approval for Sohonos (palovarotene) for FOP, but its development and launch were complicated by safety concerns, particularly skeletal growth effects in pediatric patients, resulting in a boxed warning on the product label. The failure of fidrisertib further underscores the scientific and clinical challenges of developing effective therapies for this devastating condition.
Despite this setback, Ipsen reaffirmed its commitment to supporting the FOP community and advancing scientific knowledge that might inform future therapies.
References
Ipsen update on Phase II FALKON trial in patients with ultra-rare bone disease, fibrodysplasia ossificans progressiva (FOP), 19 December 2025, https://www.ipsen.com/press-release/ipsen-update-on-phase-ii-falkon-trial-in-patients-with-ultra-rare-bone-disease-fibrodysplasia-ossificans-progressiva-fop-3208286/
A Study to Assess the Effectiveness and Safety of 2 Dosage Regimens of Oral Fidrisertib (IPN60130) for the Treatment of Fibrodysplasia Ossificans Progressiva (FOP). (FALKON), ClinicalTrials.gov ID NCT05039515, https://clinicaltrials.gov/study/NCT05039515
4 years later, Ipsen’s ‘de-risked’ rare disease drug Sohonos finally gains FDA approval, 17 August 2025, Fierce Pharma, https://www.fiercepharma.com/pharma/four-years-later-ipsens-de-risked-asset-finally-gains-fda-approval