Written By: Pharmacally Medical News Desk
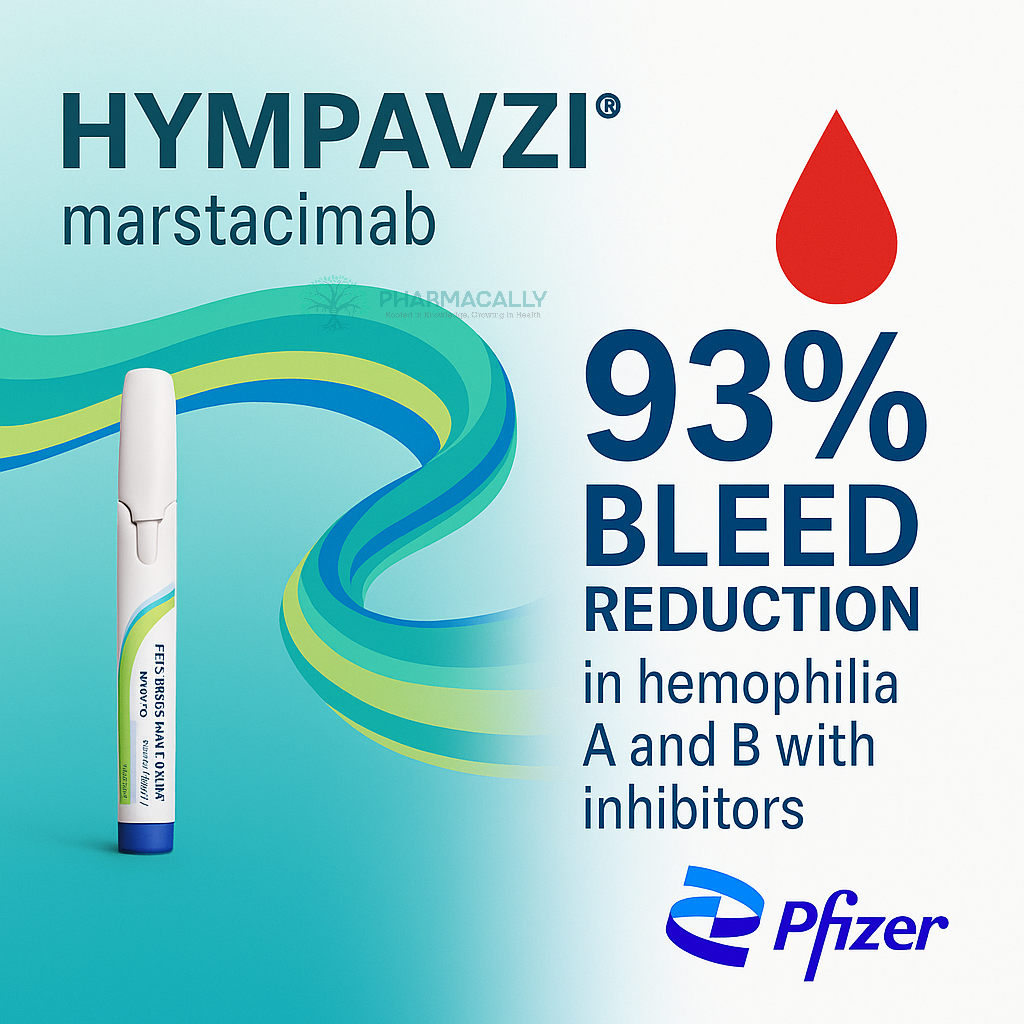
Pfizer announced new Phase 3 data showing that HYMPAVZI (Marstacimab) achieved a 93% reduction in treated annualized bleeding rate compared with on-demand bypassing agents in adults and adolescents with hemophilia A or B who have inhibitors. The findings highlight a meaningful advance for a population with limited treatment options due to resistance to standard factor replacement therapies.
Brief Study Design
The Phase 3 BASIS trial (NCT03938792) was an open-label, multicenter study enrolling 48 adolescents and adults with severe hemophilia A or moderately severe hemophilia B with inhibitors. Participants first underwent a 6-month observational period on standard on-demand bypassing therapy, followed by a 12-month active treatment phase with weekly HYMPAVZI.
Dosing included a 300 mg loading injection, then 150 mg subcutaneously once weekly, with an option to escalate to 300 mg weekly if needed.
Endpoints and Key Results
Primary Endpoint: Treated Annualized Bleeding Rate (ABR)
HYMPAVZI: 1.39 bleeds/year
On-demand therapy: 19.78 bleeds/year
Relative reduction: 93%
The median ABR was zero on HYMPAVZI, compared with 16.42 on prior therapy. Results were consistent across hemophilia types, age groups, and regions.
Secondary Endpoints:
HYMPAVZI showed superiority across all bleeding-related outcomes:
Spontaneous bleed ABR: 0.87 vs 15.27
Joint bleed ABR: 1.10 vs 15.15
Target joint bleed ABR: 0.79 vs 6.42
Total treated/untreated ABR: 4.36 vs 27.29
Quality of Life:
Six-month data showed improvements in Haem-A-QoL physical health and total scores.
Safety Profile
Safety findings were consistent with the known profile of anti-TFPI therapy. HYMPAVZI is given as a once-weekly subcutaneous injection with no need for routine lab monitoring. Patients may still require factor VIII or IX products for breakthrough bleeds and should discuss surgical plans, pregnancy, clot risk, and other medical conditions with their doctors.
Michael Vincent, MD, PhD noted that the data show HYMPAVZI has the potential to deliver meaningful bleed control with simple weekly dosing for patients with inhibitors, and said Pfizer aims to make this option available as part of its long-term commitment to advancing hemophilia care.
Davide Matino, MD, MSc highlighted that inhibitor development remains a major challenge and that the trial results suggest HYMPAVZI could become a safe and effective weekly therapy that reduces bleeding and improves quality of life for these patients.
About HYMPAVZI® (Marstacimab)
HYMPAVZI operates via a unique anti-TFPI mechanism distinct from factor VIII and factor IX replacement therapies. Rather than replacing insufficient clotting factors, HYMPAVZI is specifically designed to inhibit tissue factor pathway inhibitor (TFPI), one of the body’s natural mechanisms that obstruct the initiation of blood coagulation. By targeting Kunitz 2 unit 2 of TFPI, HYMPAVZI assists in restoring equilibrium between bleeding and clot formation.
The HYMPAVZI has received regulatory approvals in more than 40 countries for eligible patients living with hemophilia A without factor VIII inhibitors or hemophilia B without factor IX inhibitors. HYMPAVZI offers a subcutaneous treatment option with once-weekly dosing and minimal preparation required for administration, representing the first anti-TFPI approved in the U.S. and EU for hemophilia A or B treatment.