Written By: Pharmacally Medical News Desk
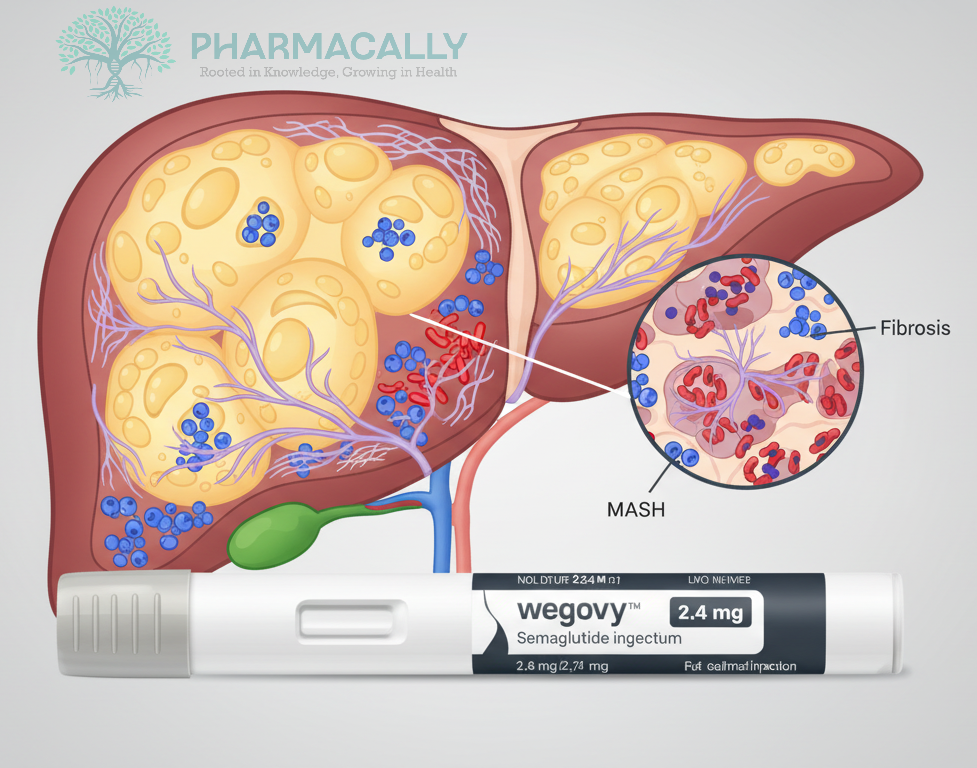
Novo Nordisk has revealed new evidence showing that Wegovy® (Semaglutide 2.4 mg), a once-weekly subcutaneous injectable, offers liver health-related benefits in adults with Metabolic Dysfunction-Associated Steatohepatitis (MASH) who have moderate to advanced liver scarring (fibrosis). These benefits appear to be partially independent of weight loss, expanding the therapeutic promise of Semaglutide beyond its established role in weight management.
Post Hoc Analysis of the ESSENCE Phase 3 Trial
This insight comes from a recent post hoc analysis of the ongoing ESSENCE phase 3 trial, which Novo Nordisk presented at the 76th annual American Association for the Study of Liver Diseases (AASLD) Liver Meeting 2025. The ESSENCE trial evaluates Semaglutide 2.4 mg in patients with biopsy-confirmed MASH and liver fibrosis stages F2 or F3.
The analysis examined liver outcomes across different weight loss thresholds (≤2%, ≤5%, ≤7%, and >7%) over 72 weeks. Remarkably, even participants with low levels of weight loss (≤2%) who received Semaglutide experienced significantly greater improvements in liver injury markers and liver scarring resolution compared to placebo recipients.
Key Findings: Liver Benefits beyond Weight Loss
Semaglutide treatment was associated with a marked reduction in liver injury (steatohepatitis) and improvements in liver fibrosis regardless of the amount of weight lost.
The greatest observed effect was on alanine aminotransaminase (ALT), a key enzyme indicative of liver injury, with patients achieving ≤7% weight loss showing a significantly greater reduction in ALT levels compared to placebo.
At the low weight loss threshold (≤2%), 48.4% of Semaglutide-treated patients showed improvement in liver injury resolution versus 25.8% in the placebo group
These results suggest semaglutide beneficial effects on liver health in MASH may be mediated through mechanisms partly independent of weight reduction.
Clinical and Scientific Implications
MASH is a progressive liver disease affecting over 250 million people globally and is characterized by irreversible liver scarring and risk of liver failure if untreated. The new findings highlight semaglutide multifaceted profile offering therapeutic potential in liver disease management beyond weight-related effects.
Professor Philip Newsome, co-chief investigator of the ESSENCE trial and director at King’s College London, emphasized the clinical importance of these findings, remarking that the data “add new layers to our understanding of MASH, which often coexists with systemic diseases including cardiometabolic disorders.”
Martin Holst Lange, Chief Scientific Officer at Novo Nordisk, noted that “even at low levels of weight loss, people with MASH receiving Semaglutide 2.4 mg had greater improvements in liver health parameters than those receiving placebo,” reinforcing the drug’s potential role in improving liver outcomes for this challenging patient population.
About the ESSENCE Trial
The ESSENCE trial (NCT04822181)is a pivotal ongoing phase 3 studies that aims to establish the efficacy and safety of Semaglutide 2.4 mg in adults with MASH complicated by moderate-to-advanced liver fibrosis. The initial part of the trial focuses on evaluating liver histology improvements at 72 weeks while the second part, still ongoing, assesses long-term clinical outcomes related to liver-related events through 240 weeks.
At summary, the latest post hoc analysis from the ESSENCE trial robustly supports the liver health benefits of Novo Nordisk’s Wegovy® (Semaglutide 2.4 mg) in adults with MASH with fibrosis. These benefits extend beyond the effects of weight loss alone, potentially changing the therapeutic landscape for MASH patients who currently face limited treatment options.
References
Novo Nordisk’s Wegovy® (semaglutide 2.4 mg) was associated with liver health-related benefits not solely based on weight loss in adult patients with MASH with liver scarring, according to a new post hoc analysis, 10 November 2025, Novo Nordisk, https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=916455
Research Study on Whether Semaglutide Works in People With Non-alcoholic Steatohepatitis (NASH) (ESSENCE), ClinicalTrials.gov ID NCT04822181, https://clinicaltrials.gov/study/NCT04822181
Arun J. Sanyal et al, Phase 3 Trial of Semaglutide in Metabolic Dysfunction–Associated Steatohepatitis, N Engl J Med 2025;392:2089-2099, VOL. 392 NO. 21, DOI: 10.1056/NEJMoa2413258