Written By: Pharmacally Medical News Desk
Novo Nordisk has announced highly promising headline results from a Phase 2 clinical trial evaluating amycretin, a next-generation unimolecular dual agonist targeting GLP-1 and amylin receptors, in people with type 2 diabetes. The trial demonstrated a significant weight loss of up to 14.5% at 36 weeks with the once-weekly subcutaneous formulation of amycretin, alongside substantial HbA1c reduction a key marker of blood glucose control with 89.1% of participants achieving HbA1c levels below 7%.
The multinational, randomized, placebo-controlled trial involved 448 participants with inadequate glycemic control on metformin, with or without SGLT2 inhibitors. Participants received either escalating doses of subcutaneous amycretin weekly or daily oral amycretin for 36 weeks. Weight loss with the subcutaneous doses reached up to 14.5%, compared to around 2.6% for placebo, while the oral amycretin group lost up to 10.1% of body weight from baseline, compared to 2.5% in the placebo group. HbA1c reductions were dose-dependent, with a maximum decrease of about 1.8% in the subcutaneous group and 1.5% in the oral group.
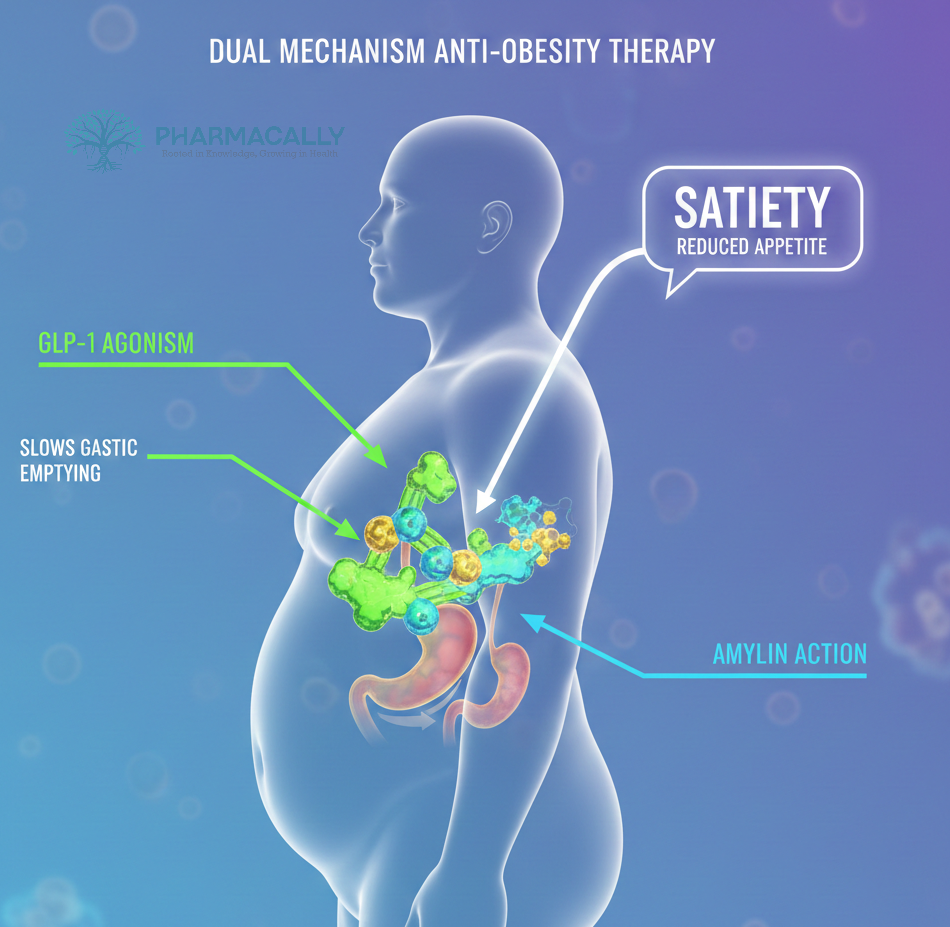
Amycretin dual action combines the beneficial effects of GLP-1 receptor agonism, which enhances insulin secretion and reduces appetite, and amylin receptor activation, which also modulates satiety and glucose metabolism. This complementary biology positions amycretin as a potential best-in-class therapy, especially as it offers both injectable and oral administration options, improving treatment flexibility and adherence.
In the evolving landscape of dual-agonist therapies targeting obesity and type 2 diabetes, CagriSema a fixed-dose combination of the GLP-1 receptor agonist Semaglutide and the amylin analog cagrilintide has also shown promising efficacy in clinical trials. While CagriSema combines two distinct molecules, amycretin represents an innovative unimolecular approach by simultaneously activating both GLP-1 and amylin receptors in a single compound. This unique design of amycretin may provide improved convenience and potentially enhanced tolerability, positioning it as a next-generation alternative in the treatment of metabolic diseases.”
Martin Holst Lange, Chief Scientific Officer and Executive Vice President of Research and Development at Novo Nordisk, commented, “We are very encouraged by the phase 2 data with amycretin in people with type 2 diabetes – the first time amycretin has been evaluated in this population. The data further validate the potential best-in-class profile of amycretin. Amycretin is built on the complementary biology of GLP-1 and amylin, and we are looking forward to bringing amycretin into an extensive phase 3 development program across multiple indications in 2026”
Safety and tolerability data aligned with expectations for therapies involving incretin and amylin pathways, with mostly mild to moderate gastrointestinal side effects being the most common. There was no observed plateau in weight loss by week 36, indicating sustained efficacy. Novo Nordisk is preparing to advance amycretin into an extensive Phase 3 development program for type 2 diabetes and potentially other indications in 2026.
References
Novo Nordisk phase 2 trial with amycretin reports significant weight loss and HbA1c reduction in type 2 diabetes, 25 November 2025, Novo Nordisk, https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=916463
CagriSema: A Powerful New Weight Loss and Diabetes Combination Therapy Emerges from REDEFINE-2 Trial, Pharmacally, 14 June 2025, https://pharmacally.com/cagrisema-a-powerful-new-weight-loss-and-diabetes-combination-therapy-emerges-from-redefine-2-trial/