Written By: Pharmacally Medical News Desk
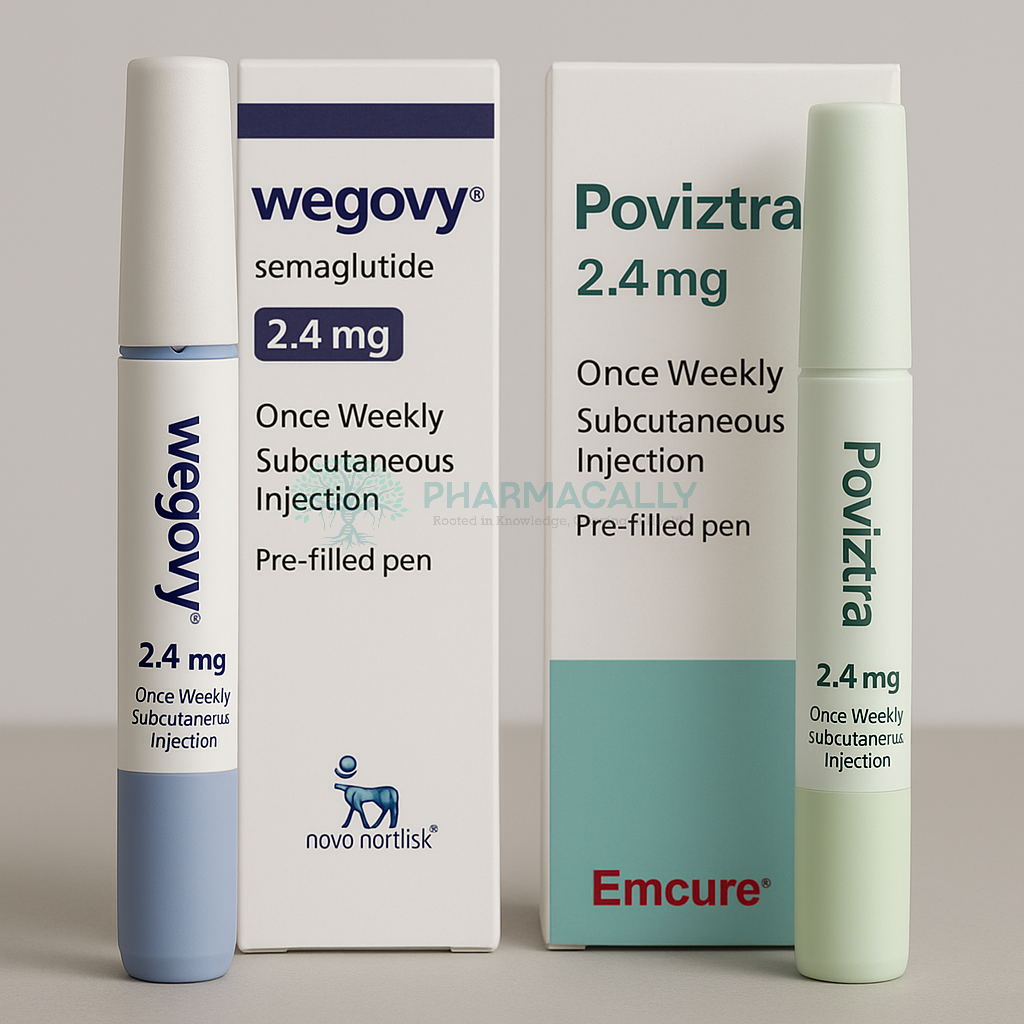
Novo Nordisk India has entered into a strategic partnership with Emcure Pharmaceuticals to distribute and market semaglutide injection 2.4 mg under the brand name Poviztra across India. This collaboration aims to extend availability and accessibility of this leading weight management therapy beyond Novo Nordisk’s existing reach, particularly targeting regions and pharmacies that were previously underserved.
Emcure Pharma, headquartered in Pune and ranked among India’s top pharmaceutical companies, becomes the first Indian firm to hold exclusive rights to distribute and commercialize Poviztra considered a second brand of Novo Nordisk’s flagship weight loss drug Wegovy. Launched in India in June 2025, Wegovy demonstrated strong clinical efficacy, with about one in three patients losing more than 20% of their body weight during trials.
The partnership leverages Emcure’s extensive marketing and distribution network to broaden access to semaglutide, a GLP-1 receptor agonist approved as a once-weekly injection that supports chronic weight management and reduces cardiovascular risk in overweight or obese adults with heart disease. Emcure’s strong foothold in diverse geographic and therapeutic segments such as women’s health and gastroenterology positions the company to reach patients across urban and rural areas.
Pricing details for Poviztra (semaglutide) have not been officially disclosed, but Novo Nordisk’s similar product Wegovy is priced at ₹26,015 for a month’s supply of the 2.4 mg dose in India.
Satish Mehta, CEO of Emcure Pharma, expressed enthusiasm about being the first Indian company to commercialize this globally trusted molecule, emphasizing Emcure’s ability to deliver the treatment to patients who need it most. Jay Thyagarajan, Senior Vice President at Novo Nordisk Asia Pacific, highlighted that the collaboration combines Novo Nordisk’s innovation with Emcure’s distribution strength to scale the medicine’s reach beyond metro cities and premium clinics.
With India facing a significant obesity challenge over 600 million people affected by general or abdominal obesity this partnership stands as a key step in expanding treatment options for weight management. The semaglutide injection, administered via a user-friendly pen device in doses ranging from 0.25 mg to 2.4 mg, is positioned to meet growing demand in both major cities and smaller towns.
Novo Nordisk and Emcure have emphasized strict measures and legal actions to curb illegal access and counterfeit sales of semaglutide in India, including collaboration with regulatory authorities and online marketplaces to remove unauthorized listings and safeguard patient safety.
This Novo Nordisk-Emcure alliance follows Lilly and Cipla’s collaboration to market Mounjaro (tirzepatide) as Yurpeak illustrating a trend where global pharmaceutical companies partner with Indian firms to navigate complex distribution environments and regulatory systems, ultimately broadening patient access to cutting-edge therapies.
Key Points
Novo Nordisk partners with Emcure Pharma to launch Poviztra, semaglutide 2.4 mg injection, as a second brand of Wegovy in India.
Emcure holds exclusive marketing and distribution rights, focusing on expanding access beyond Novo Nordisk’s existing network.
Semaglutide supports weight loss and reduces cardiovascular risks (MACE) in obese patients.
The partnership targets underserved regions and aims to meet India’s growing obesity burden.
Emcure’s deep market presence strengthens the drug’s reach in both urban and rural India.
Reflects a strategic trend of global pharma collaborating with Indian companies for chronic disease management.
References
Novo Nordisk India partners with Emcure Pharma to commercialize Poviztra® in India as a second brand of Wegovy®, Emcure, 10 November 2025, available at https://www.emcure.com/media-center/
Anti-obesity segment sees heavy-weights Novo Nordisk and Eli Lilly slug it out, Businessline, 10 November 2025, https://www.thehindubusinessline.com/companies/novo-nordisk-inks-collaboration-with-emcure-to-sell-on-its-weightloss-and-diabetes-drug-wegovy/article70261658.ece
Novo Nordisk partners with Emcure to launch weight-loss drug under new brand in India, The Economic Times, 10 November 2025, https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/novo-nordisk-emcure-to-launch-weight-loss-drug-under-new-brand-in-india/articleshow/125217308.cms?from=mdr
Mounjaro Now Cipla’s Yurpeak: A New Chapter for Tirzepatide in India, 24 October 2025, Pharmacally, https://pharmacally.com/mounjaro-now-ciplas-yurpeak-a-new-chapter-for-tirzepatide-in-india/
Long-Term SELECT Trial: Semaglutide Cuts Cardiovascular Events by 20% beyond Weight Loss, 26 October 2025, Pharmacally, https://pharmacally.com/long-term-select-trial-semaglutide-cuts-cardiovascular-events-by-20-beyond-weight-loss/
Semaglutide Battle – Latest Court Ruling Explained, Dr. Rupali Rana Arya, Linkedin, 24 July 2025, https://www.linkedin.com/pulse/semaglutide-battle-latest-court-ruling-explained-arya-zkvgf/
Can the Weight Loss Indication of Semaglutide and Tirzepatide Be Revoked in India? Understanding the Recent PIL against GLP-1 Drugs, 08 July 2025, Pharmacally, https://pharmacally.com/can-the-weight-loss-indication-of-semaglutide-and-tirzepatide-be-revoked-in-india-understanding-the-recent-pil-against-glp-1-drugs/