Written By: Abhinay Wadekar, BPharm
Reviewed By: Pharmacally Editorial Team
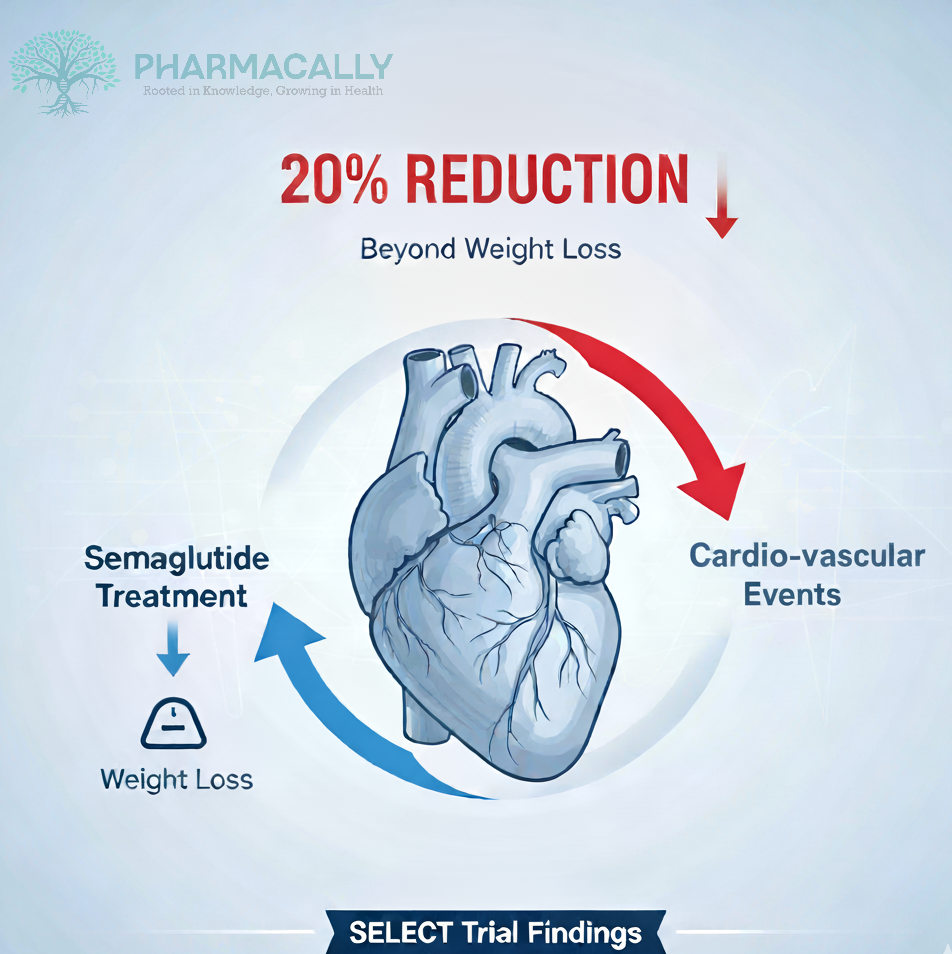
Semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA) and member of WHO essential medicine’s list has revolutionized obesity management not only through substantial weight reduction but also through cardiovascular protection. The SELECT trial, published in The Lancet (October 22, 2025), provides a landmark analysis confirming that the cardioprotective benefits of semaglutide extend beyond weight loss alone.
The SELECT Trial: Study Overview
The SELECT trial (NCT03574597) enrolled 17,604 participants aged 45 years and above across 41 countries and 804 sites. All participants had overweight or obesity (BMI ≥27 kg/m²) and pre-existing cardiovascular disease (CVD) but notably no diabetes. Participants were randomly assigned in a 1:1 ratio to receive either semaglutide 2.4 mg once weekly or a placebo, along with standard cardiovascular care. The study aimed to see how often major cardiovascular events occurred, including non-fatal heart attack, non-fatal stroke, or death from cardiovascular causes.
The trial followed participants for almost four years. On average, those taking semaglutide received treatment for 33 months. The results showed a 20% lower risk of major cardiovascular events compared to the placebo group.
Baseline Body Weight and MACE Risk
Baseline analysis revealed that higher BMI and waist circumference correlated with greater cardiovascular risk, confirming obesity’s mechanistic role through hemodynamic, metabolic, and inflammatory pathways. However, the cardiovascular benefit of semaglutide was consistent across all body weight subgroups, showing no significant interaction between BMI or waist measurements and treatment effect.
The SELECT trial showed that for every 5 kg lower baseline body weight, patients treated with semaglutide had a 4% lower risk of major cardiovascular events (hazard ratio 0.96). Similarly, a 5 cm smaller waist circumference at baseline was linked to a 4% lower risk of such events (HR 0.96). These results suggest that semaglutide’s cardiovascular benefits are consistent regardless of a person’s starting body weight or fat distribution. This means its ability to reduce cardiovascular risk likely involves mechanisms beyond simple weight or fat loss.
Weight Loss and Cardiovascular Outcomes
Although semaglutide led to an average placebo-adjusted weight loss of 8.5% (Semaglutide arm lost 8.5% more body weight than those who took the placebo), its cardiovascular risk reduction was not linked to how much weight patients lost. Early weight loss at week 20 did not predict later cardiovascular benefits (HR 0.95), suggesting that semaglutide’s heart-protective effects may work through mechanisms independent of blood sugar control.
In the placebo group, patients who lost more than 5% of their body weight unexpectedly showed higher rates of major cardiovascular events, likely due to unintentional weight loss from underlying illness. In contrast, patients taking semaglutide maintained strong cardiovascular protection, regardless of how much weight they lost.
Waist Circumference Reduction: A Key Marker
Waist circumference, a measure of visceral fat, showed a modest but meaningful link to cardiovascular outcomes in the SELECT trial. Patients who reduced their waist size by week 20 experienced fewer major cardiovascular events (MACE), with a hazard ratio of 0.91. Further analysis suggested that about 33% of semaglutide MACE risk reduction was due to this decrease in waist circumference, with an adjusted HR of 0.86 after accounting for waist changes. This indicates that while reducing central fat contributes to the cardiovascular benefits, most of semaglutide protective effects likely come from other mechanisms, such as anti-inflammatory actions and improvements in endothelial function.
Mechanisms beyond Weight and Adiposity
The study suggests that semaglutide protects the heart through several mechanisms. It improves blood vessel function and reduces vascular inflammation, lowers blood pressure and harmful lipids, and acts on GLP-1 receptors in the brain to influence inflammation and autonomic control. These multiple effects show that semaglutide does more than help with weight loss it can modify the course of cardiometabolic disease, positioning GLP-1 receptor agonists as therapies that protect the heart and metabolism and not just obesity.
Safety Profile`
Semaglutide showed a consistent safety profile across all weight and waist change categories, with similar rates of cardiac and non-cardiac side effects. Notably, participants in the placebo group who lost a large amount of weight unexpectedly had higher non-cardiovascular mortality, suggesting that weight loss from semaglutide is controlled and physiologically safer.
Clinical and Policy Implications
The SELECT trial challenges the idea of using GLP-1 receptor agonists only for weight loss. Semaglutide heart-protective benefits appear across all BMI ranges and do not depend on weight loss. This supports expanding its use to all overweight or obese patients with atherosclerotic cardiovascular disease, revising BMI-based prescription limits to focus on reducing cardiovascular risk, and positioning GLP-1RAs as an important part of secondary prevention for heart disease.
Key Expert Opinions
Professor Subodh Verma, lead Canadian investigator for the SELECT program, presented an extended sub-analysis at the ACC 2025 conference, confirming semaglutide 22% reduction in total cardiovascular events, including recurrent heart attacks and strokes. He stated, “Semaglutide 2.4 mg not only reduces the first event risk but also lowers the cumulative cardiovascular burden making it a foundational therapy for secondary prevention in obesity-related cardiovascular disease”
Vince Lamanna, President of Novo Nordisk Canada, commented that “these results expand the meaning of cardiovascular care by integrating obesity treatment as a proven strategy to prevent future events.” He highlighted that Health Canada’s decision to approve semaglutide 2.4 mg for patients with established CVD and overweight or obesity was “the first global regulatory recognition of obesity therapy as a cardiovascular indication.
Findings from the SELECT trial highlight semaglutide strong cardiovascular protection that remains effective regardless of weight or fat reduction. This repositions GLP-1 receptor agonists like semaglutide 2.4 mg as true cardiometabolic therapies rather than just anti-obesity drugs. By lowering the risk of major cardiovascular events by 20% in overweight or obese patients without diabetes, semaglutide stands out as a breakthrough treatment for cardiovascular risk reduction.
References
Deanfield, John et al., Semaglutide and cardiovascular outcomes by baseline and changes in adiposity measurements: a prespecified analysis of the SELECT trial, The Lancet, Published on October 22, 2025, DOI: 10.1016/S0140-6736(25)01375-3
Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT), ClinicalTrials.gov ID NCT03574597, https://clinicaltrials.gov/study/NCT03574597
Semaglutide 2.4 mg reduces burden of total cardiovascular events in people with established cardiovascular disease and overweight or obesity, Novo Nordisk, 31 March 2025, https://www.novonordisk.ca/content/dam/nncorp/ca/en/press-releases/2025/acc-2025-select-total-events-canada-english.pdf