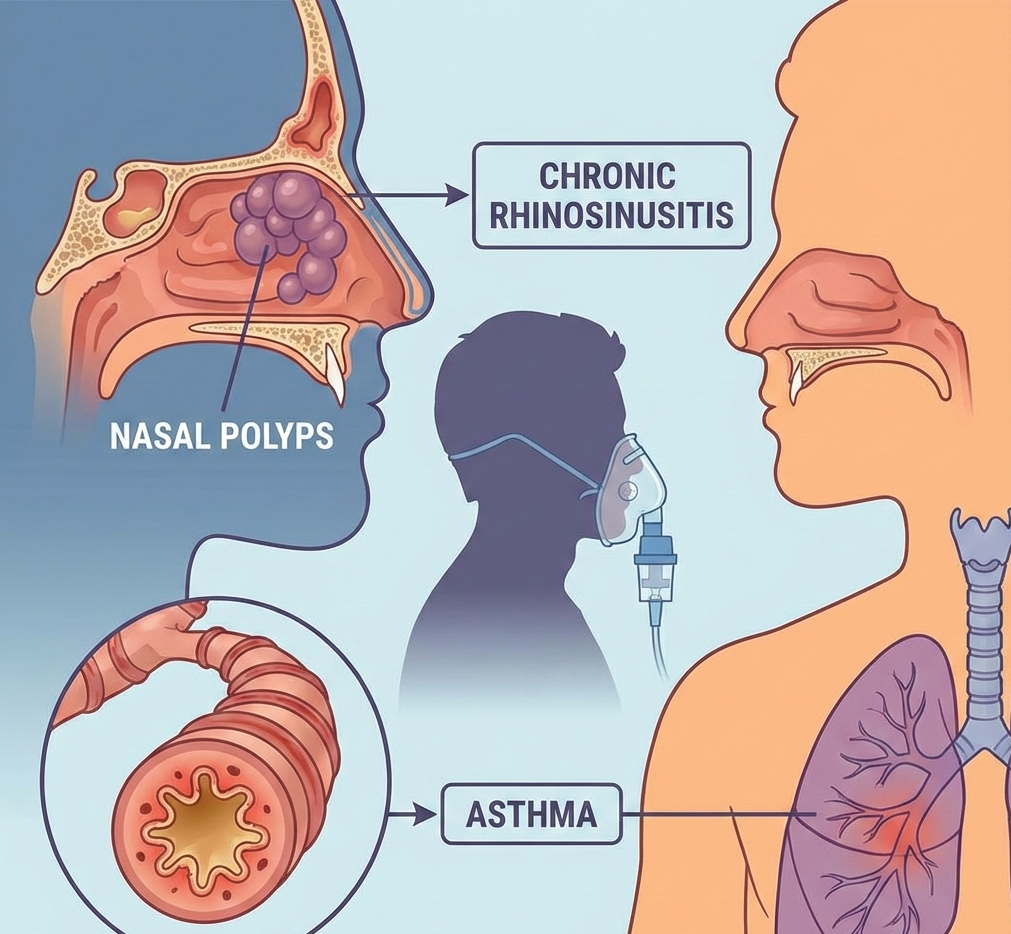
Exdensur (depemokimab) has been approved in Japan for severe asthma and chronic rhinosinusitis with nasal polyps, becoming the country’s first ultra-long-acting biologic with twice-yearly dosing. The decision is based on the same global data reviewed in earlier U.S. and U.K. approvals, with Japan-specific guidance to follow from the PMDA.
Written By: Pharmacally Medical News Desk
Japan’s Ministry of Health, Labour and Welfare has approved Exdensur (depemokimab) for severe or refractory bronchial asthma that remains uncontrolled on current treatments, and for chronic rhinosinusitis with nasal polyps (CRSwNP) in patients who do not respond adequately to standard therapy.
This approval makes Exdensur the first and only ultra-long-acting biologic available in Japan for these conditions, with dosing scheduled just twice a year alongside standard care.
What this approval means for Japan
Severe asthma continues to drive frequent exacerbations, hospital visits, and healthcare costs. Many patients with CRSwNP also live with persistent nasal blockage, loss of smell, infections, and repeated surgeries despite treatment. Access to a twice-yearly biologic may help simplify long-term management and improve control for patients who struggle with existing options.
Country-specific prescribing guidance, dosing, and safety precautions will be published through the Pharmaceuticals and Medical Devices Agency (PMDA).
Basis for the decision
The PMDA approval draws on the same pivotal data package reviewed by regulators in the United States and the United Kingdom, including the SWIFT-1 SWIFT-2 and ANCHOR-1 ANCHOR-2 phase III programs and the global safety database. These studies showed sustained benefit when Exdensur was added to standard therapy in both severe asthma and CRSwNP.
For readers who want full trial results, efficacy outcomes, and detailed safety information, we will direct them to our earlier comprehensive coverage of the U.S. and U.K. approvals.
Expert perspective
Commenting on the decision, Kaivan Khavandi, SVP and Global Head of Respiratory, Immunology and Inflammation R&D at GSK, said the approval in Japan could help establish a new treatment approach for patients with severe asthma or CRSwNP. He noted that Exdensur delivers sustained suppression of type 2 inflammation with only two doses a year, giving physicians an ultra-long-acting option to help reduce asthma attacks and relieve CRSwNP symptoms.
Part of a growing global rollout
Japan becomes the third major market to authorize depemokimab, following approvals in the United States and the United Kingdom. The medicine has also received a positive opinion in the European Union and remains under review in several other countries, including China.
Reference
Exdensur (depemokimab) approved in Japan for severe asthma and chronic rhinosinusitis with nasal polyps, 06 January 2026, https://www.gsk.com/en-gb/media/press-releases/exdensur-depemokimab-approved-in-japan/
Placebo-controlled Efficacy and Safety Study of GSK3511294 (Depemokimab) in Participants with Severe Asthma with an Eosinophilic Phenotype (SWIFT-1), ClinicalTrials.gov ID NCT04719832, https://clinicaltrials.gov/study/NCT04719832
A Study of GSK3511294 (Depemokimab) in Participants With Severe Asthma With an Eosinophilic Phenotype (SWIFT-2), ClinicalTrials.gov ID NCT04718103, https://clinicaltrials.gov/study/NCT04718103
David J. Jackson et al, Twice-Yearly Depemokimab in Severe Asthma with an Eosinophilic Phenotype, N Engl J Med 2024;391:2337-2349, DOI: 10.1056/NEJMoa2406673
Efficacy and Safety of Depemokimab (GSK3511294) in Participants with Chronic Rhinosinusitis with Nasal Polyps (ANCHOR-1), ClinicalTrials.gov ID NCT05274750, https://clinicaltrials.gov/study/NCT05274750
Efficacy and Safety of Depemokimab (GSK3511294) in Participants with Chronic Rhinosinusitis with Nasal Polyps (ANCHOR-2) (ANCHOR-2), ClinicalTrials.gov ID NCT05281523, https://clinicaltrials.gov/study/NCT05281523
Gevaert, Philippe et al, Efficacy and safety of twice per year depemokimab in chronic rhinosinusitis with nasal polyps (ANCHOR-1 and ANCHOR-2): phase 3, randomised, double-blind, parallel trials, The Lancet, Volume 405, Issue 10482, 911 – 926, DOI:10.1016/S0140-6736(25)00197-7