FDA grants Priority Review to Vera Therapeutics’ atacicept for IgA nephropathy, with a PDUFA date of July 7, 2026. The therapy targets BAFF and APRIL and is being evaluated for its potential to slow kidney damage in adults with IgAN.
Written By: Pharmacally Medical News Desk
The U.S. FDA has accepted the Biologics License Application (BLA) for atacicept and granted Priority Review for the treatment of adults with immunoglobulin A nephropathy (IgAN).
The application was submitted under the Accelerated Approval pathway, and the agency has set a Prescription Drug User Fee Act (PDUFA) target action date of July 7, 2026. If approved, atacicept could become the first B‑cell modulator in IgAN to target both B‑cell Activating Factor (BAFF) and A Proliferation‑Inducing Ligand (APRIL) and may be available as a once‑weekly, subcutaneous autoinjector for at‑home administration.
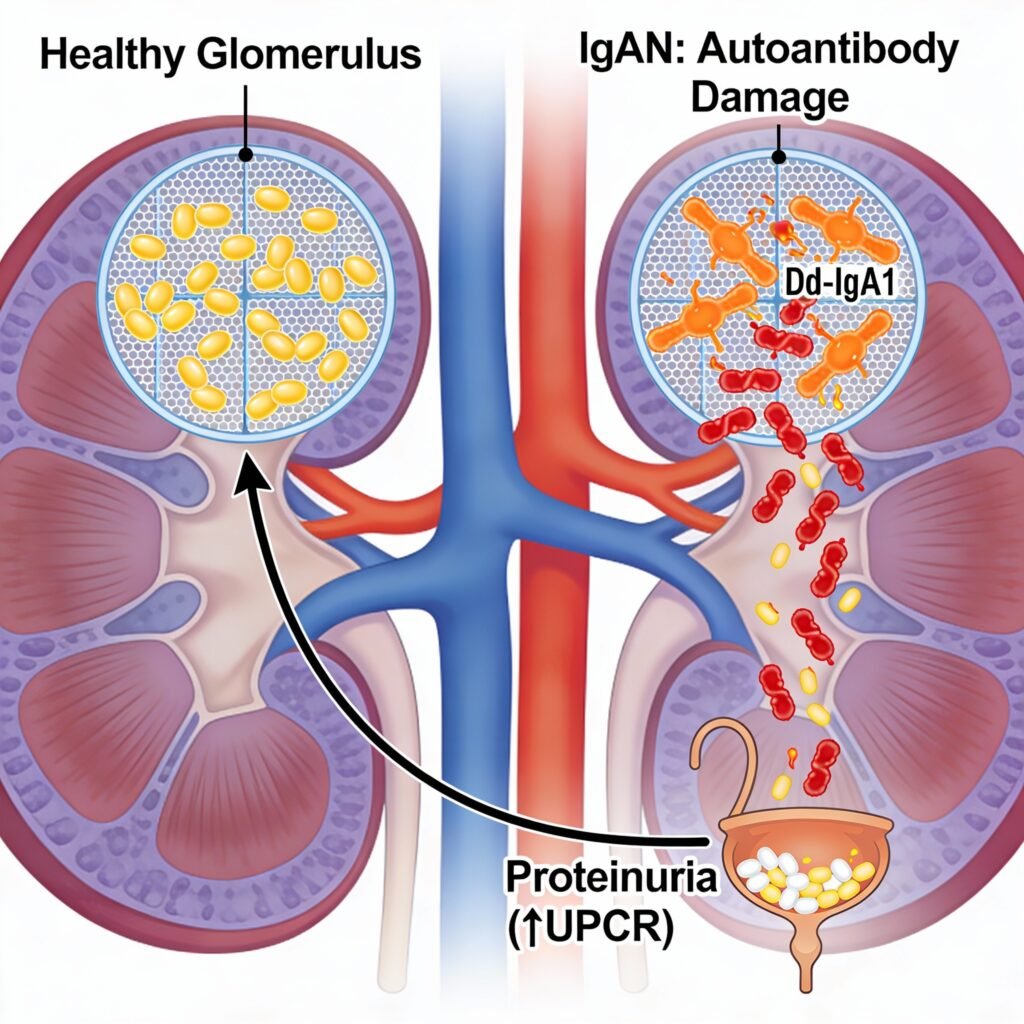
Atacicept is designed to intervene early in the IgAN disease process by inhibiting BAFF and APRIL, key cytokines that drive B‑cell survival and autoantibody production. By blocking these pathways, the therapy aims to reduce pathogenic autoantibodies that damage the glomerular filtration barrier, with the goal of slowing disease progression and preserving kidney function.
The Priority Review is supported by interim data from the ongoing Phase 3 ORIGIN 3 trial (NCT04716231). At week 36, atacicept achieved a 46% reduction in proteinuria from baseline and a statistically significant 42% reduction in urine protein‑to‑creatinine ratio (UPCR) versus placebo, with safety findings generally comparable to placebo across the clinical program.
These results build on Phase 2b data demonstrating clinically meaningful reductions in proteinuria, improvements in biomarkers such as galactose‑deficient IgA1 (Gd‑IgA1) and hematuria, and stabilization of estimated glomerular filtration rate (eGFR) over longer follow‑up. The ORIGIN 3 trial continues to evaluate long‑term kidney outcomes, with key eGFR data expected in 2027.
Marshall Fordyce, founder and CEO of Vera Therapeutics, highlighted the potential for atacicept to change how IgAN is managed, noting that dual BAFF/APRIL targeting addresses upstream biological drivers of disease rather than focusing solely on downstream inflammation. The FDA’s Breakthrough Therapy Designation and Priority Review underscore both the high unmet need for disease‑modifying therapies in IgAN and the strength of the emerging clinical evidence for atacicept.
Beyond IgAN, atacicept is being investigated across a broader spectrum of immune‑mediated kidney diseases, including primary membranous nephropathy, focal segmental glomerulosclerosis (FSGS), and minimal change disease within the PIONEER program.
Vera is also extending access through the ORIGIN Extend study while collecting longer‑term safety and efficacy data, positioning atacicept as a potential platform therapy for B‑cell–mediated kidney disorders.
IgA nephropathy is a serious, progressive autoimmune kidney disease that can lead to end‑stage kidney disease in up to half of affected patients over time. Current therapies primarily slow progression rather than directly targeting the underlying immune dysregulation, so a treatment capable of modifying disease biology could help shift the treatment paradigm and reduce the long‑term burden of dialysis and transplantation.
With the FDA review now underway, upcoming regulatory milestones and confirmatory outcomes data from ORIGIN 3 will be closely watched. If approved, atacicept could offer patients a convenient, self‑administered therapy that targets disease biology while clinicians monitor for durable effects on kidney function over time.
References
Vera Therapeutics Announces U.S. FDA Granted Priority Review to Biologics License Application for Atacicept for Treatment of Adults with IgA Nephropathy, 07 January 2026, https://ir.veratx.com/node/10316/pdf
Vera Therapeutics Announces Atacicept Achieved 46% Proteinuria Reduction in ORIGIN Phase 3 Trial in Adults with IgA Nephropathy, 02 June 2025, https://ir.veratx.com/news-releases/news-release-details/vera-therapeutics-announces-atacicept-achieved-46-proteinuria
Atacicept in Subjects with IgA Nephropathy (ORIGIN 3), ClinicalTrials.gov ID NCT04716231, https://clinicaltrials.gov/study/NCT04716231
Lafayette R et al, A Phase 3 Trial of Atacicept in Patients with IgA Nephropathy. N Engl J Med. 2025 Nov 6. Epub ahead of print. PMID: 41196369. https://doi.org/10.1056/nejmoa2510198
Vera Therapeutics Announces Positive ORIGIN Phase 3 Data for Atacicept in IgA Nephropathy Presented at ASN Kidney Week 2025 and Published in the New England Journal of Medicine, 06 November 2025, https://ir.veratx.com/news-releases/news-release-details/vera-therapeutics-announces-positive-origin-phase-3-data