Written by: Pharmacally Medical News Desk
On September 9, 2025 the U.S. Food and Drug Administration approved Inlexzo (gemcitabine intravesical system; Janssen Biotech, Inc.) for adults with Bacillus Calmette-Guérin (BCG)–unresponsive non-muscle-invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors. Inlexzo, formerly known as TAR-200, is a first-of-its-kind intravesical drug-releasing system (iDRS) that delivers sustained-release gemcitabine directly into the bladder. It combines device and pharmaceutical innovation. Janssen Biotech, a part of J&J’s Innovative Medicine; has been working on this device-drug combination since 2019 is granted this approval.
Disease
BCG remains the standard intravesical therapy for high-risk NMIBC, including CIS. Patients who fail BCG (so-called BCG-unresponsive disease) face limited options: radical cystectomy is recommended for many, while systemic or alternative intravesical therapies have variable efficacy and tolerability. A treatment that achieves high CR rates and durable responses without removing the bladder would therefore be an important addition for patients ineligible for or declining cystectomy. The FDA approval positions Inlexzo as an option for this specific, high-need population.
What is Inlexzo and How it Works
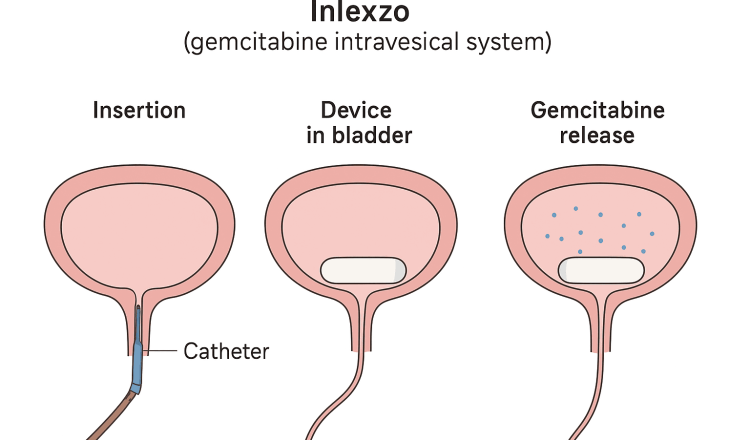
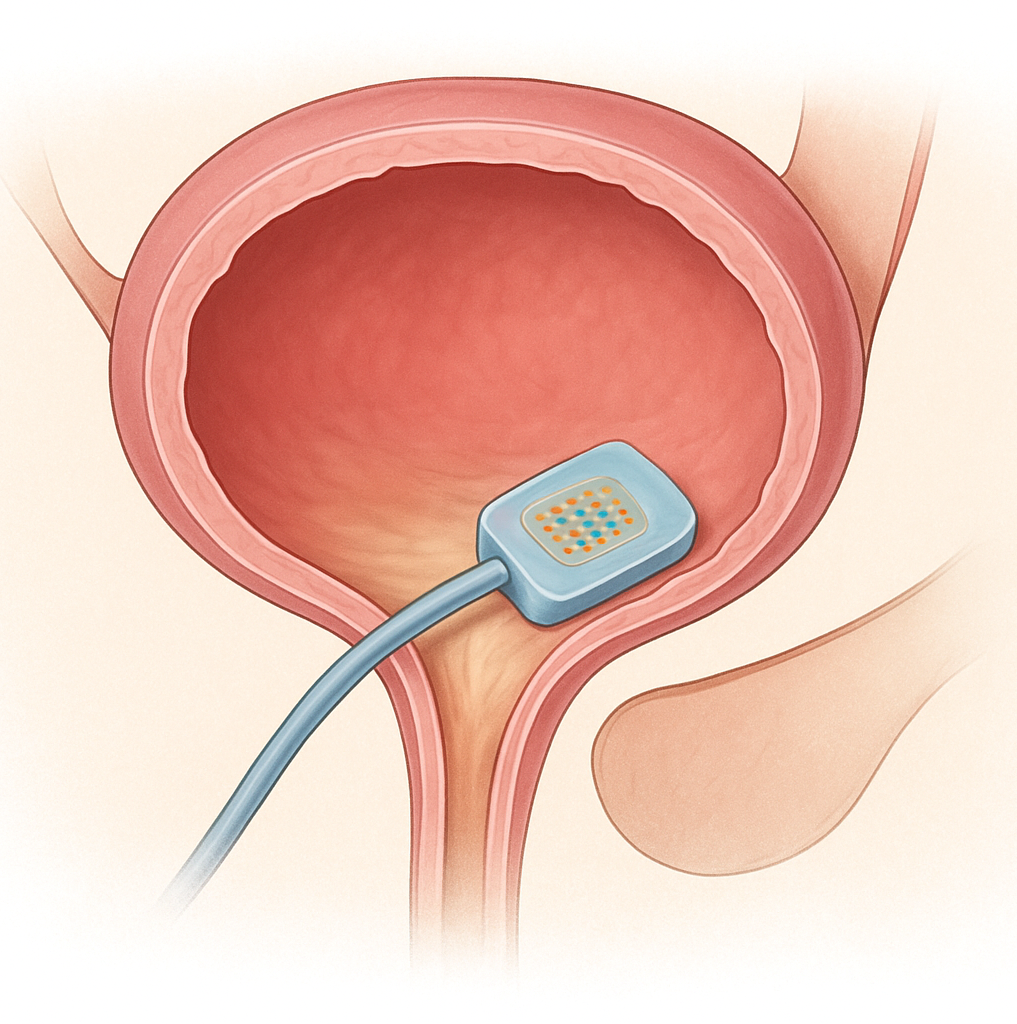
Inlexzo is a gemcitabine intravesical system a device designed to remain in the bladder and slowly release gemcitabine directly to the urothelium over an extended period.
Preparation and Insertion: The device is preloaded with gemcitabine in a polymer-based reservoir. A urinary catheter and stylet, co-packaged with Inlexzo, are used for placement. The urologist inserts the catheter into the bladder and uses the stylet to advance the device into position. Once deployed, the device remains inside the bladder for the treatment cycle (about 3 weeks per cycle).
Drug Release Mechanism: The system is engineered as an intravesical drug-releasing system (iDRS). Through a controlled polymeric release mechanism, small, steady doses of gemcitabine diffuse directly into the bladder wall (urothelium) over time. This creates sustained local exposure at therapeutic levels, unlike standard intravesical instillation where drug is flushed out quickly with urine.
Action on Tumor Cells: Gemcitabine, a nucleoside analog, gets incorporated into DNA during replication. This causes chain termination and inhibition of DNA synthesis, leading to apoptosis of rapidly dividing tumor cells in the bladder lining. Because delivery is local, systemic exposure is minimal, reducing risks of systemic chemotherapy-related toxicities.
Removal: After the scheduled dwell period (typically ~3 weeks), the device is removed via cystoscopy or catheter retrieval. Patients may receive multiple cycles, depending on clinical response and tolerability.
Clinical Evidence
The approval of Inlexzo (gemcitabine intravesical system) was primarily supported by Cohort 2 of the SunRISe-1 trial (NCT04640623), a phase 2b, open-label, multicohort study designed to evaluate TAR-200/Inlexzo in patients with high-risk, BCG-unresponsive non-muscle invasive bladder cancer (NMIBC). Cohort 2 specifically enrolled adults with carcinoma in situ (CIS), with or without papillary tumors, who were ineligible for or declined radical cystectomy. The primary endpoint was complete response (CR) rate, centrally assessed. Key secondary endpoints included duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety outcomes.
Results from Cohort 2 demonstrated a centrally confirmed CR rate of 82.4% among evaluable patients, showing that the majority achieved disease eradication following treatment. Importantly, about 51–53% of responders maintained CR for at least 12 months, meeting the FDA’s threshold for durable benefit in this disease setting. The median DoR among responders was 25.8 months indicating long-lasting disease control in many patients. Although the study was single-arm and non-randomized, the robust response rates and durability in a population with limited therapeutic options provided convincing evidence for regulatory approval.
Warning and Precaution
Inlexzo should not be used in patients with a perforated or compromised bladder, as this may lead to systemic gemcitabine exposure and severe toxicity. Patients must be counseled that delaying radical cystectomy when CIS persists carries a real risk of disease progression to muscle-invasive or metastatic cancer. The device is MR Conditional and can only undergo MRI under the strict conditions outlined in the prescribing information. Because gemcitabine can cause embryo-fetal harm, women of reproductive potential should use effective contraception during treatment and for six months after device removal, and men with female partners should use contraception for three months after removal.
Safety Profile
The safety analysis of Cohort 2 indicated that Inlexzo was generally well tolerated, with adverse events largely confined to the urinary tract. The most frequent treatment-emergent adverse events included urinary tract infection, urinary frequency, dysuria, hematuria, and local bladder discomfort. Most of these were low-grade and manageable with standard interventions. Serious adverse events and systemic toxicities were uncommon, consistent with the localized drug delivery mechanism of the intravesical system, which minimizes systemic gemcitabine exposure. Overall, the benefit–risk profile was considered favorable compared with existing bladder-sparing alternatives for BCG-unresponsive NMIBC.
FDA Stance
This application was granted priority review. The gemcitabine intravesical system received breakthrough therapy designation. Healthcare professionals should report all serious adverse events suspected to be associated with the use of any medicine and device to FDA’s Med Watch Reporting System or by calling 1-800-FDA-1088.
Reference
FDA approves gemcitabine intravesical system for non-muscle invasive bladder cancer, USFDA, 09 Sept 2025, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-gemcitabine-intravesical-system-non-muscle-invasive-bladder-cancer
Inlexzo, Full Prescribing Information, https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INLEXZO-pi.pdf
U.S. FDA approval of INLEXZO™ (gemcitabine intravesical system) set to transform how certain bladder cancers are treated, 09 Sept 2025, https://www.jnj.com/media-center/press-releases/u-s-fda approval-of-inlexzo-gemcitabine-intravesical-system-set-to-transform-how-certain-bladder-cancers-are-treated