Written By: Pharmacally Medical News Desk
The U.S. Food and Drug Administration (FDA) seized many fake Ozempic (Semaglutide) 1 mg injection pens on December 5, 2025. These counterfeits got into the real U.S. drug supply chain without going through Novo Nordisk’s approved sellers.
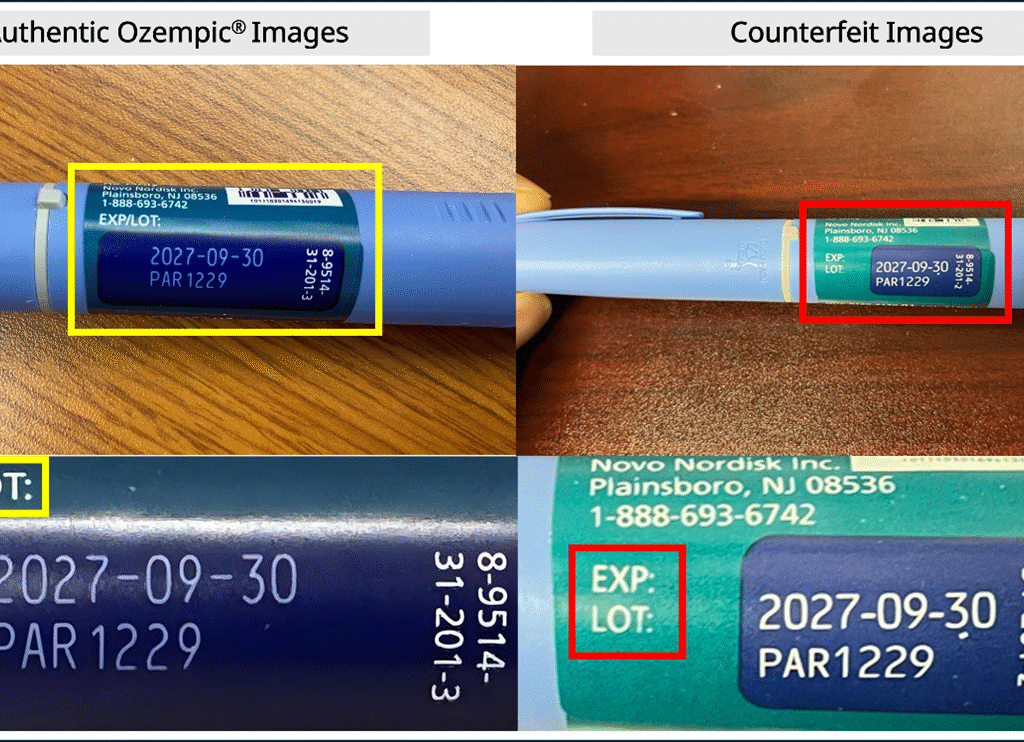
The fake pens use the real lot number PAR1229, but the EXP/LOT text sits on the left side of the expiration date and lot number on the label. On genuine pens, this text appears above them. Patients who got PAR1229 from Novo Nordisk’s Patient Assistance Program can keep using it, as those are confirmed real.
This is the newest case in FDA’s ongoing checks on fake Ozempic. It follows earlier seizures, like hundreds of units on April 9, 2025 (lot PAR0362, serial starting 51746517) and thousands in December 2023 (lot NAR0074, serial 430834149057).
Novo Nordisk told the FDA about the December 2025 problem on November 18, 2025. This led to lab tests on the fakes’ ingredients and dangers, including risks from non-sterile needles like those found in the December 2023 case
Official Guidelines for Patient, Retailers and Healthcare professionals
Patients should get Ozempic only with a valid prescription from a licensed pharmacy in their state. This will make sure the medicine is real. Before using an Ozempic pen, check the label closely for fake signs, like EXP/LOT text in the wrong spot or odd lot numbers such as PAR1229.
Pharmacies, drug wholesalers, and doctors need to check Ozempic shipments against Novo Nordisk’s list of approved sellers. This wills stops fake products from reaching people. Do not sell or give out any suspect batches from FDA warnings, like PAR1229 with wrong labeling.
If consumers have any questions about a batch, contact Novo Nordisk directly using their official contact details. Patients, pharmacies, wholesalers, and healthcare professionals should report any fake pens, adverse reactions, or shady sellers to their local FDA complaint coordinator or visit the FDA website for further details.
Reporting and Next Steps
Healthcare providers and patients must report adverse events through FDA’s MedWatch program online or fax at 1-800-FDA-0178. FDA collaborates with Novo Nordisk and federal partners to remove fakes, emphasizing risks like unknown ingredients, contamination, or infection from unsterile components. Ongoing testing will clarify safety profiles of seized units.
Global Scope of Counterfeit Ozempic and GLP-1RA
Counterfeit Ozempic and other GLP-1 receptor agonist (RA) drugs like Semaglutide represent a worldwide problem, not limited to the U.S. The European Medicines Agency (EMA) warned EU patients and professionals about falsified Ozempic pens found at wholesalers in countries like Austria and Germany, urging checks on suspicious offers.
The World Health Organization (WHO) also issued alerts on fake Semaglutide batches detected in Brazil, the UK, and the U.S. since 2023, noting risks from incorrect ingredients or insulin substitutes across all regions.
Australia’s Therapeutic Goods Administration (TGA) seized counterfeit Ozempic pens containing insulin glulisine instead of Semaglutide, while the UK’s MHRA flagged falsified products entering legal supply chains.
References
FDA warns consumers not to use counterfeit Ozempic (Semaglutide) found in U.S. drug supply chain, 05 December 2025, https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-use-counterfeit-ozempic-semaglutide-found-us-drug-supply-chain
Novo Nordisk warns consumers about counterfeit Ozempic® (Semaglutide) injection 1 mg in the US, 06 December 2025, https://www.novonordisk-us.com/media/news-archive/news-details.html?id=916466
EMA Issues Safety Warning on Sudden Rise in Illegal Semaglutide, Liraglutide, and Tirzepatide Sale in European Union, 04 September 2025, https://pharmacally.com/ema-issues-safety-warning-on-sudden-rise-in-illegal-semaglutide-liraglutide-and-tirzepatide-sale-in-european-union/
Counterfeit Medicine, 08 December 2025, https://www.fda.gov/drugs/buying-using-medicine-safely/counterfeit-medicine