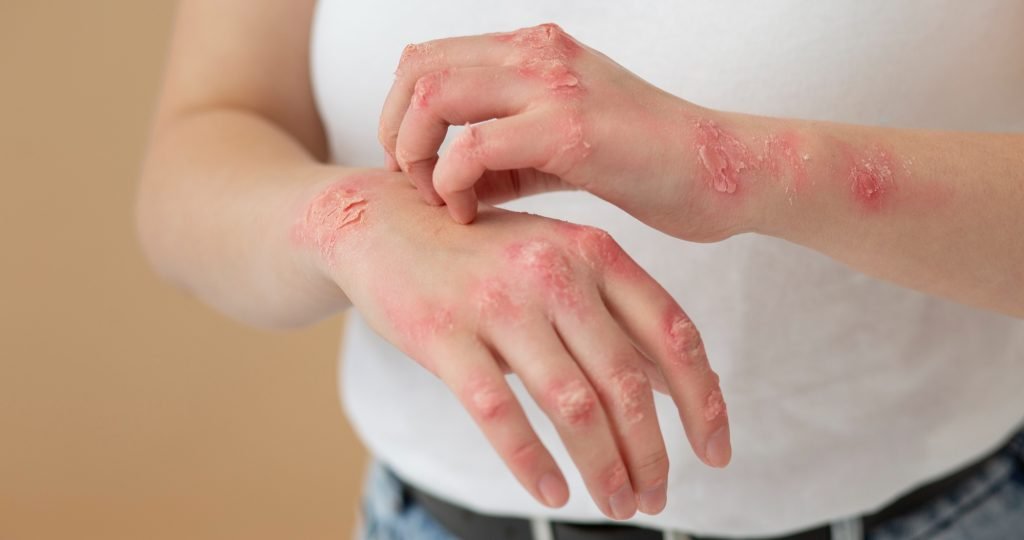
At-a-Glance
- Eli Lilly has reported positive topline trial results for Taltz and Zepbound drugs in psoriasis patients with obesity or overweight.
- Phase 3b trial (NCT06588283) tested Taltz + Zepbound vs. Taltz alone in 274 adults with moderate-to-severe plaque psoriasis and obesity/overweight.
- Primary endpoint met: 27.1% achieved PASI 100 + ≥10% weight loss vs. 5.8% on Taltz monotherapy (p<0.001).
- Key secondary: 40.6% reached PASI 100 on combo vs. 29.0% on Taltz alone (40% relative increase, p<0.05).
Written By: Pavan Kumar Chikkula PharmD
Reviewed By: Pharmacally Editorial Team
Eli Lilly and Company announced positive topline results from the TOGETHER-PsO Phase 3b trial, showing that combining Taltz (ixekizumab) with Zepbound (tirzepatide) significantly outperforms Taltz alone for adults with moderate-to-severe plaque psoriasis and obesity or overweight plus at least one weight-related comorbidity. At 36 weeks, the duo met the primary endpoint and all key secondary endpoints, achieving better skin clearance and weight loss in a high-burden patient group.
Breakthrough Primary Endpoint Results
TOGETHER-PsO (NCT06588283) is a 52-week, randomized, open-label study. Participants got subcutaneous Taltz ± Zepbound, plus diet/exercise counselling. Detailed 36-week results will be published in a peer-reviewed journal and shared with regulators.
The trial’s primary goal was complete skin clearance (Psoriasis Area and Severity Index [PASI] 100) plus at least 10% weight loss. Impressively, 27.1% of patients on Taltz + Zepbound hit this mark, compared to just 5.8% on Taltz monotherapy (p<0.001).
This first-of-its-kind study enrolled 274 participants with tough-to-treat disease: average BMI over 39 kg/m² (9-10 kg/m² higher than typical Phase 3 psoriasis biologic trials), ~25% body surface area affected, and 97% with high-impact areas like face, scalp, or genitals involved.
A key secondary endpoint showed Taltz + Zepbound boosted PASI 100 rates by 40% relative to Taltz alone (40.6% vs. 29.0%; p<0.05). These gains underscore how addressing obesity with Zepbound lightens psoriasis burden, especially since higher BMI often worsens outcomes.
Safety
Adverse events were mild to moderate, matching each drug’s profile. Common ones in the combo arm: nausea, diarrhea, constipation, injection site reactions, dosing errors, vomiting, dizziness (≥5%). Taltz arm: injection site reactions, dosing errors, nasopharyngitis.
“These results represent far more than a clinical milestone they show what becomes possible when we address both diseases simultaneously,” said Adrienne Brown, Lilly Immunology president.
Principal investigator Mark Lebwohl, M.D., from Icahn School of Medicine at Mount Sinai, added: “Treating psoriasis and obesity together significantly improved outcomes, reinforcing psoriasis as an obesity-related condition.”
This trial builds on Taltz + Zepbound data from the TOGETHER-PsA (NCT06588296) psoriatic arthritis trial, positioning Taltz as the only biologic with evidence for combo use with an incretin therapy in these populations.
Taltz (ixekizumab) is a monoclonal antibody that selectively binds to IL-17A, a key pro-inflammatory cytokine driving psoriasis. By neutralizing IL-17A, it blocks its interaction with the IL-17 receptor, reducing inflammation, keratinocyte proliferation, and chemokine release to clear plaques in psoriasis, psoriatic arthritis, ankylosing spondylitis, and more.
Zepbound (tirzepatide), a dual GIP/GLP-1 receptor agonist, activates these gut hormone receptors to suppress appetite, slow gastric emptying, and lower calorie intake, aiding significant weight loss; it’s FDA-approved for obesity/overweight management and moderate-to-severe OSA in obesity.
Why This Matters for Patients
In the U.S., 61% of psoriasis patients have obesity or overweight with comorbidities, yet treatments often ignore this overlap. Psoriasis and obesity share inflammatory pathways, but guidelines urge integrated care. TOGETHER-PsO proves concomitant therapy works in real-world high-BMI cases.
This trial signals a shift toward holistic care, potentially transforming outcomes for psoriasis patients with obesity.
Reference
Lilly’s Taltz (ixekizumab) and Zepbound (tirzepatide) used together delivered superior efficacy in first-of-its-kind Phase 3b trial for adults with psoriasis and obesity or overweight, 18 February 2026, Lilly’s Taltz (ixekizumab) and Zepbound (tirzepatide) used together delivered superior efficacy in first-of-its-kind Phase 3b trial for adults with psoriasis and obesity or overweight | Eli Lilly and Company
Ixekizumab Concomitantly Administered With Tirzepatide in Adults With Moderate-to-Severe Plaque Psoriasis and Obesity or Overweight (TOGETHER-PsO), ClinicalTrials.gov ID NCT06588283, Study Details | NCT06588283 | Ixekizumab Concomitantly Administered With Tirzepatide in Adults With Moderate-to-Severe Plaque Psoriasis and Obesity or Overweight | ClinicalTrials.gov
Ixekizumab Concomitantly Administered with Tirzepatide in Adults with Psoriatic Arthritis and Obesity or Overweight (TOGETHER-PsA), ClinicalTrials.gov ID NCT06588296, https://clinicaltrials.gov/study/NCT06588296
Lilly’s Taltz Plus Zepbound Delivers Superior Outcomes in Phase 3b Psoriatic Arthritis Trial With Obesity, 09 January 2026, https://pharmacally.com/lillys-taltz-plus-zepbound-delivers-superior-outcomes-in-phase-3b-psoriatic-arthritis-trial-with-obesity/
