Written By: Sheetal Barbade, BPharm
Reviewed By: Pharmacally Editorial Team
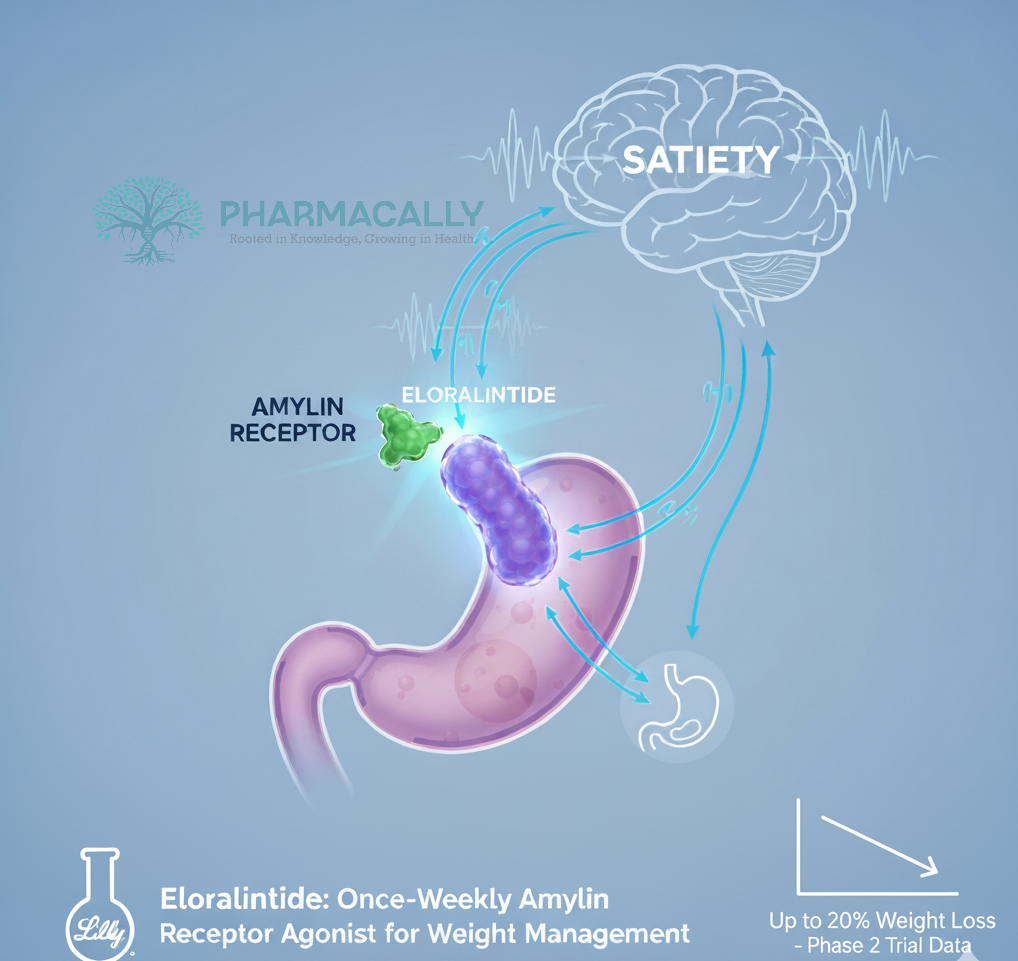
Eli Lilly recently announced encouraging results from a Phase 2 clinical trial evaluating eloralintide, a selective amylin receptor agonist, as a novel treatment for adults with obesity or overweight. The 48-week randomized, placebo-controlled trial demonstrated meaningful and dose-dependent weight loss, with reductions ranging from 9.5% to 20.1% at the highest dose, alongside favorable safety. These findings underscore eloralintide potential as a distinct therapeutic mechanism for obesity management, expanding options beyond current incretin-based drugs.
Study Design
The Phase 2 trial (NCT06230523) was a multicenter, double-blind, randomized; placebo-controlled study enrolling 263 adults aged 18 to 75 years with obesity or overweight plus at least one obesity-related comorbidity excluding those with type 2 diabetes. Participants were randomized to receive once-weekly subcutaneous injections of eloralintide at doses of 1 mg, 3 mg, 6 mg, or 9 mg, or dose escalation regimens of 3–9 mg or 6–9 mg, or placebo. The primary endpoint was percentage change in body weight from baseline at 48 weeks.
Results and Endpoints
At 48 weeks, all eloralintide treatment arms significantly outperformed placebo, which showed only a 0.4% mean weight reduction. Weight loss at the highest dose (9 mg) reached up to 20.1%, with other doses showing dose-dependent responses (9.5% to 20%). Secondary endpoints also improved significantly, including body mass index reduction and favorable changes in cardiometabolic risk factors such as waist circumference, blood pressure, lipid profiles, glycemic control, and markers of inflammation.
Safety and Tolerability
Eloralintide was generally well tolerated. The most common adverse events were mild to moderate gastrointestinal symptoms (notably nausea) and fatigue. These were dose-related and less frequent with slower dose escalations. Lower dose arms (1 mg and 3 mg) had adverse event rates similar to placebo, supporting a favorable safety profile.
Table: Phase 2 Weight Loss Results of Eli Lilly’s Eloralintide by Dose Escalation
Eloralintide Dose (mg) | Mean % Change in Body Weight | Mean Weight Loss (kg) | Mean Weight Loss (lbs) |
1 | -9.5% | -10.2 | -22.5 |
3 | -12.4% | -13.3 | -29.3 |
3 / 6 / 9 (dose escalation) | -16.4% | -17.8 | -39.2 |
6 | -17.6% | -18.7 | -41.2 |
6 / 9 (dose escalation) | -19.9% | -21.0 | -46.3 |
9 | -20.1% | -21.3 | -47.0 |
Placebo | -0.4% | -0.2 | -0.4 |
About Amylin
Amylin, a hormone co-secreted with insulin by pancreatic beta cells, regulates appetite and gastric emptying. Amylin receptor agonists represent an emerging class of obesity treatments distinct from glucagon-like peptide-1 (GLP-1) receptor agonists, with the potential for complementary or alternative therapeutic options for weight management.
About Eloralintide
Eloralintide (development code LY3841136) is Eli Lilly’s once-weekly, long-acting, selective amylin receptor agonist being developed for chronic weight management, now advancing from Phase 2 to planned Phase 3 trial. Eloralintide was engineered as a potent, long-acting analog of human amylin with high selectivity for the amylin receptor, especially AMY1R, to drive satiety and reduce energy intake while aiming to mitigate nausea seen with less selective ligands. Eloralintide demonstrated preferential activation of human AMY1R over calcitonin receptor (CTR) and AMY3R in vitro and produced less conditioned taste avoidance than the nonselective comparator cagrilintide in rat studies, suggesting improved GI tolerability potential.
Amylin receptors (AMYR), especially AMY1R is particularly implicated in satiety signaling in the hindbrain and hypothalamus. Eloralintide selectively and potently activates AMY1R with much lower activity at CTR and reduced activity at AMY3R, consistent with focused amylin-pathway agonism enhance satiety and reduced caloric intake. Enhances satiety, reduces energy intake, slows gastric emptying are subsequently supports weight loss; Lilly communications emphasize decreased caloric intake mediated by satiety as the likely primary driver.
Future Steps
Eli Lilly plans to initiate Phase 3 clinical trials of eloralintide monotherapy for obesity treatment by the end of 2025. Concurrently, a Phase 2 trial (NCT06603571) is ongoing to evaluate eloralintide in combination with tirzepatide, assessing safety, tolerability, and enhanced weight loss effects. These future studies aim to confirm long-term efficacy and safety while exploring combination treatment options to better address individual patient needs in obesity management.
Key Opinions
Dr. Liana K. Billings, MD Director of Clinical and Genetics Research in Diabetes and Cardiometabolic Disease at Endeavor Health, Skokie, Illinois, and lead author emphasized that eloralintide shows promise with a tolerability profile that does not compromise efficacy, illustrating the potential of amylin receptor agonists to expand obesity treatment options. Kenneth Custer, Ph.D., executive vice president and president of Lilly Cardiometabolic Health noted that Lilly is committed to a comprehensive obesity medicine pipeline, with eloralintide offering strong efficacy and improved tolerability as a selective amylin receptor agonist. He expressed optimism that it may serve as an alternative or complementary option to incretin therapies, with Phase 3 enrolment planned by year-end based on encouraging Phase 2 results.
References
Lilly’s selective amylin agonist, eloralintide, demonstrated meaningful weight loss and favorable tolerability in a Phase 2 study of adults with obesity or overweight, 06 November 2025, Lilly, https://investor.lilly.com/news-releases/news-release-details/lillys-selective-amylin-agonist-eloralintide-demonstrated
Briere DA, et al, Eloralintide (LY3841136), a novel amylin receptor agonist for the treatment of obesity: From discovery to clinical proof of concept. Mol Metab. 2025 Oct 16;102:102271. Doi: 10.1016/j.molmet.2025.102271. Epub ahead of print. PMID: 41109426.
Liana K Billings et al, Eloralintide, a selective amylin receptor agonist for the treatment of obesity: a 48-week phase 2, multicentre, double-blind, randomised, placebo-controlled trial, The Lancet, 2025, https://doi.org/10.1016/S0140-6736(25)02155-5.
Kirsten Dahl et al, NN1213-A Potent, Long-Acting, and Selective Analog of Human Amylin, J. Med. Chem. 2024, 67, 14, 11688–11700
A Study of LY3841136 Compared with Placebo in Adult Participants with Obesity or Overweight, ClinicalTrials.gov ID NCT06230523, https://clinicaltrials.gov/study/NCT06230523