Denali Therapeutics reports Phase 1/2 results for tividenofusp alfa (DNL310) in Hunter syndrome (MPS II), showing biomarker normalization and clinical improvements, with findings later published in NEJM.
Written By: Pharmacally Medical News Desk
Denali Therapeutics announced on December 30, 2025, results from its Phase 1/2 study of tividenofusp alfa (DNL310) in children with Hunter syndrome, also known as mucopolysaccharidosis type II (MPS II). The therapy showed strong reductions in key disease biomarkers and encouraging clinical signals across multiple domains. Treatment led to reductions and, in many cases, normalization of heparan sulfate levels, along with stabilization or improvement in adaptive behavior, cognition, hearing, and liver volume. The data come from an open-label study designed to evaluate both brain and systemic effects of therapy. These findings were later published in The New England Journal of Medicine.
The Phase 1/2 study (NCT04251026) was an open-label, single-arm trial in pediatric participants with MPS II. The primary focus was safety and tolerability, with secondary measures evaluating effects on central nervous system (CNS) and peripheral disease indicators. In the published analysis involving 47 participants, tividenofusp alfa led to:
Marked reductions in disease biomarkers
Cerebrospinal fluid (CSF) heparan sulfate (HS) levels dropped by around 91 % at 24 weeks, with reductions maintained through long-term follow-up.
Urine HS levels were reduced by approximately 88 % at 24 weeks and sustained over time.
Neurofilament light (NfL), a marker of neuronal injury, showed substantial decline by later time points, with many individuals reaching levels similar to unaffected children.
Normalization of key measures
The majority of participants achieved CSF and urine HS values within the normal range for children without MPS II after 24 weeks of treatment.
Clinical signals of benefit
Beyond biomarkers, investigators reported liver volume normalization, improvements in hearing thresholds, and gains or stabilization in adaptive behavior and cognition.
The most frequent treatment-related events were infusion-related reactions, which decreased with ongoing dosing and were generally manageable.
Mechanism of Tividenofusp Alfa
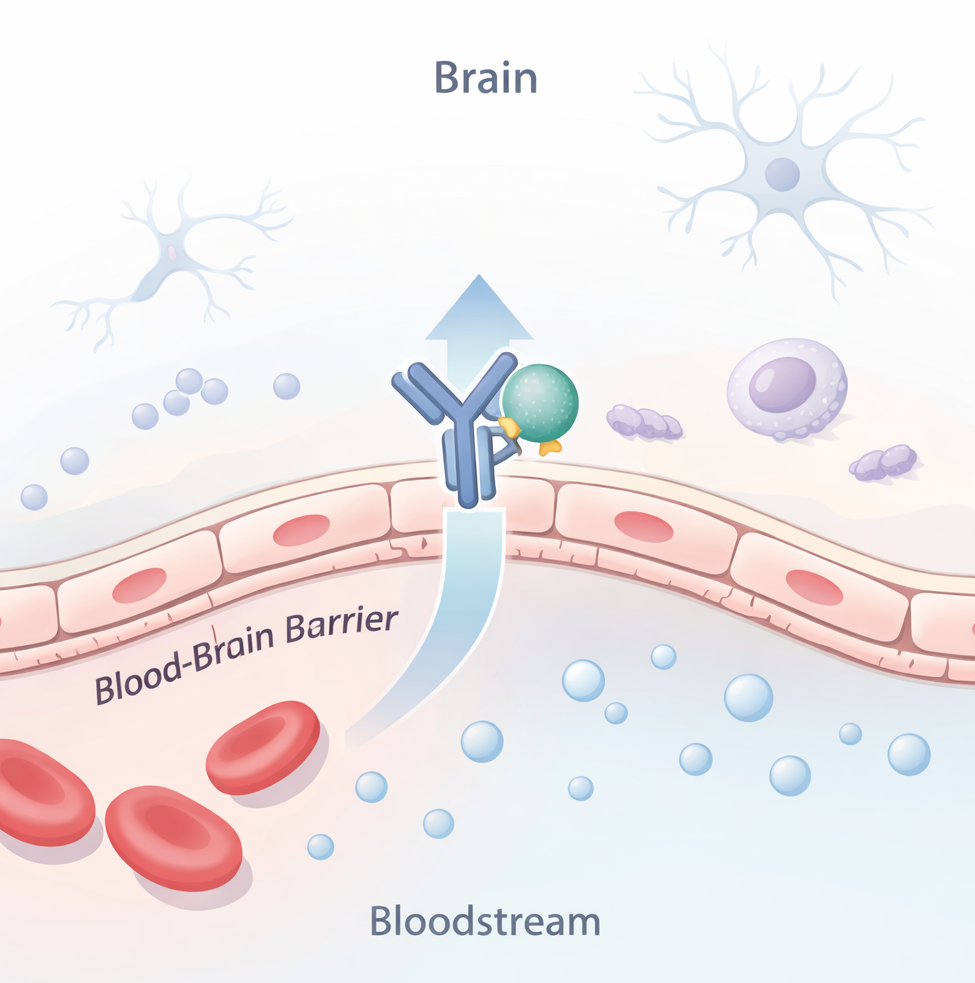
Tividenofusp alfa is a brain-penetrant enzyme replacement therapy designed to address both CNS and peripheral aspects of Hunter syndrome, a severe lysosomal storage disorder caused by mutations in the iduronate-2-sulfatase (IDS) gene. The therapy combines the IDS enzyme with Denali’s proprietary TransportVehicle platform, which aims to cross the blood-brain barrier and deliver enzyme activity throughout the body, including the brain. Traditional enzyme replacement therapies do not reach the CNS, leaving neurological symptoms largely untreated.
Understanding Hunter Syndrome
Hunter syndrome (MPS II) is a rare, X-linked lysosomal storage disorder that largely affects males. A deficiency in the IDS enzyme leads to accumulation of glycosaminoglycans (GAGs) such as heparan sulfate and dermatan sulfate in tissues throughout the body and brain. This buildup causes progressive organ dysfunction, cognitive and behavioural decline, hearing loss, joint stiffness, and other systemic complications. Standard enzyme replacement therapies help some physical symptoms but do not cross the blood-brain barrier, leaving neurological aspects untreated. There is a clear unmet need for therapies that can address the full spectrum of disease manifestations.
Denali’s TransportVehicle platform uses engineered Fc domains that engage natural receptors at the blood-brain barrier, enabling therapeutic proteins to be transported into the brain while also reaching peripheral tissues. This design allows tividenofusp alfa to address both neurological and systemic aspects of Hunter syndrome, unlike conventional enzyme replacement therapy.
Regulatory Context and Next Steps
Based on these Phase 1/2 findings, Denali has pursued regulatory pathways in key markets:
The U.S. Food and Drug Administration (FDA) has granted Priority Review, with a Prescription Drug User Fee Act (PDUFA) target action date set for April 5, 2026 as part of the ongoing biologics license application (BLA) evaluation.
The company continues to conduct a Phase 2/3 study (COMPASS) comparing tividenofusp alfa to the current standard therapy (idursulfase), which will provide confirmatory evidence to support full approval and global regulatory submissions.
Tividenofusp alfa remains investigational and has not yet been approved by any health authority.
The publication of these Phase 1/2 results in a leading NEJM highlights the potential of tividenofusp alfa to change the treatment landscape for Hunter syndrome by targeting both central and peripheral disease processes. If approved, it could become the first therapy designed to address neurological and somatic symptoms in this rare disorder.
References
The New England Journal of Medicine Publishes Phase 1/2 Study of Denali Therapeutics’ Tividenofusp Alfa (DNL310) for Hunter Syndrome (MPS II), 30 December 2025, https://investors.denalitherapeutics.com/news-releases/news-release-details/new-england-journal-medicine-publishes-phase-12-study-denali
Joseph Muenzer et al, An Intravenous Brain-Penetrant Enzyme Therapy for Mucopolysaccharidosis II, N Engl J Med 2026;394:39-50, DOI: 10.1056/NEJMoa2508681
A Study of Tividenofusp Alfa (DNL310) in Pediatric Participants with Hunter Syndrome, ClinicalTrials.gov ID NCT04251026, https://clinicaltrials.gov/study/NCT04251026