The FDA has accepted Abbisko Therapeutics’ NDA for pimicotinib for the treatment of tenosynovial giant cell tumor, supported by positive Phase III MANEUVER trial results and key global regulatory milestones.
Written By: Sana Khan Bpharm
Reviewed By: Pharmacally Editorial Team
Abbisko Therapeutics announced that the US Food and Drug Administration has accepted its New Drug Application for pimicotinib for the treatment of tenosynovial giant cell tumor. The acceptance indicates the application is sufficiently complete for a full regulatory review and marks a significant step in Abbisko’s global development plans for the therapy.
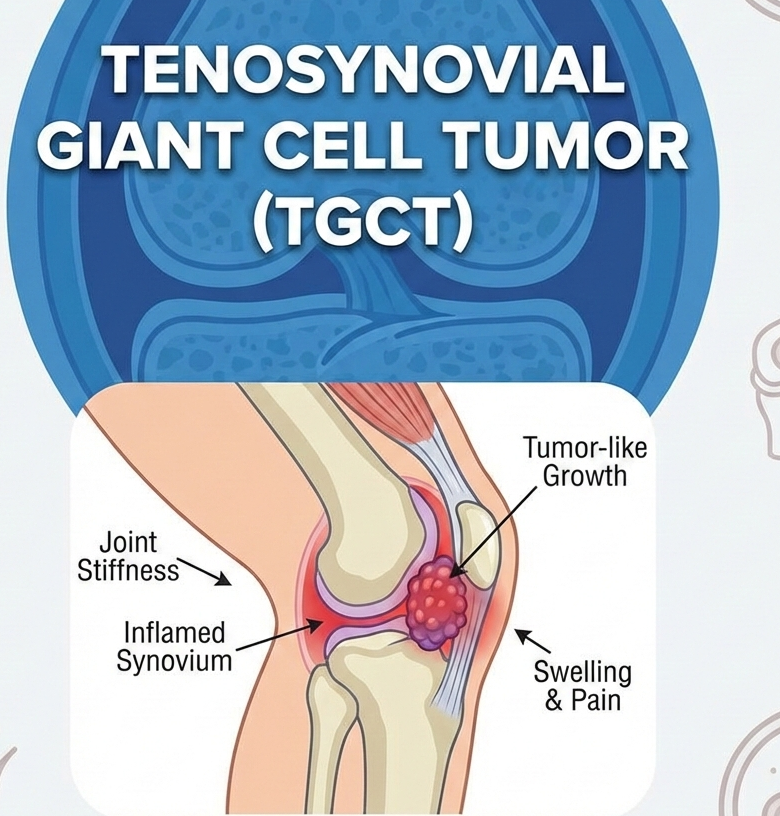
Tenosynovial giant cell tumor is a rare, locally aggressive condition driven by abnormal overproduction of colony-stimulating factor 1 (CSF1), which can lead to joint damage, pain, stiffness, and reduced mobility. Surgery remains the standard approach, but recurrence is common and some patients are not ideal surgical candidates, underscoring the need for effective systemic options.
Pimicotinib is an oral, selective inhibitor of the CSF1 receptor (CSF1R) pathway. By blocking CSF1R signaling, the drug is designed to reduce the accumulation of tumor-associated macrophages believed to drive inflammation and tissue overgrowth in TGCT. Abbisko is positioning pimicotinib as a potential disease-modifying option for patients with advanced or unresectable disease.
The FDA’s NDA acceptance is supported by efficacy and safety results from the global, multicentre, randomized, double-blind, placebo-controlled Phase III MANEUVER trial (NCT05804045). In the study, once-daily oral pimicotinib achieved a statistically significant improvement in the primary endpoint of objective response rate (ORR) at Week 25, assessed by a blinded independent review committee using RECIST v1.1 criteria. Reported results showed an ORR of 54.0% with pimicotinib versus 3.2% with placebo and nearly all patients in the pimicotinib group (92.1%) had a decrease in tumor size at the primary analysis cutoff.
The MANEUVER trial also demonstrated statistically significant and clinically meaningful benefits across key secondary endpoints, including improvements in active range of motion and physical function, along with reductions in stiffness and pain based on patient-reported outcomes. Longer-term follow-up data (median 14.3 months) indicated that ORR continued to increase over time among patients who received pimicotinib from the start of the study.
Beyond the US filing, pimicotinib has received regulatory recognition from major global agencies, including Breakthrough Therapy Designation from the FDA and PRIME Designation from the European Medicines Agency. In China, pimicotinib was approved by the National Medical Products Administration in December 2025 for adult patients with symptomatic TGCT in whom surgical resection may result in functional limitation or significant morbidity.
Abbisko has also entered into a commercial agreement with Merck KGaA, Darmstadt, Germany, under which Merck KGaA is responsible for commercialization in Greater China and holds an option for additional international commercialization rights under agreed terms. Together, these regulatory milestones and strategic partnerships support Abbisko’s broader global development strategy for pimicotinib in TGCT.
References
Abbisko Therapeutics Announces FDA Acceptance of the NDA for Pimicotinib for the Treatment of Tenosynovial Giant Cell Tumor, 13 January 2026, https://www.abbisko.com/newsDetail/234.html
Study of Pimicotinib (ABSK021) for Tenosynovial Giant Cell Tumor (MANEUVER), ClinicalTrials.gov ID NCT05804045, https://www.clinicaltrials.gov/study/NCT05804045
Niu X, Ravi V, Shan B, Guo Q, Shi H, Zou Q, Gelderblom H. MANEUVER: A Phase III study of pimicotinib to assess efficacy and safety in tenosynovial giant cell tumor patients. Future Oncol. 2024 Sep 17:1-8. Epub ahead of print. PMID: 39287124. https://doi.org/10.1080/14796694.2024.2396227