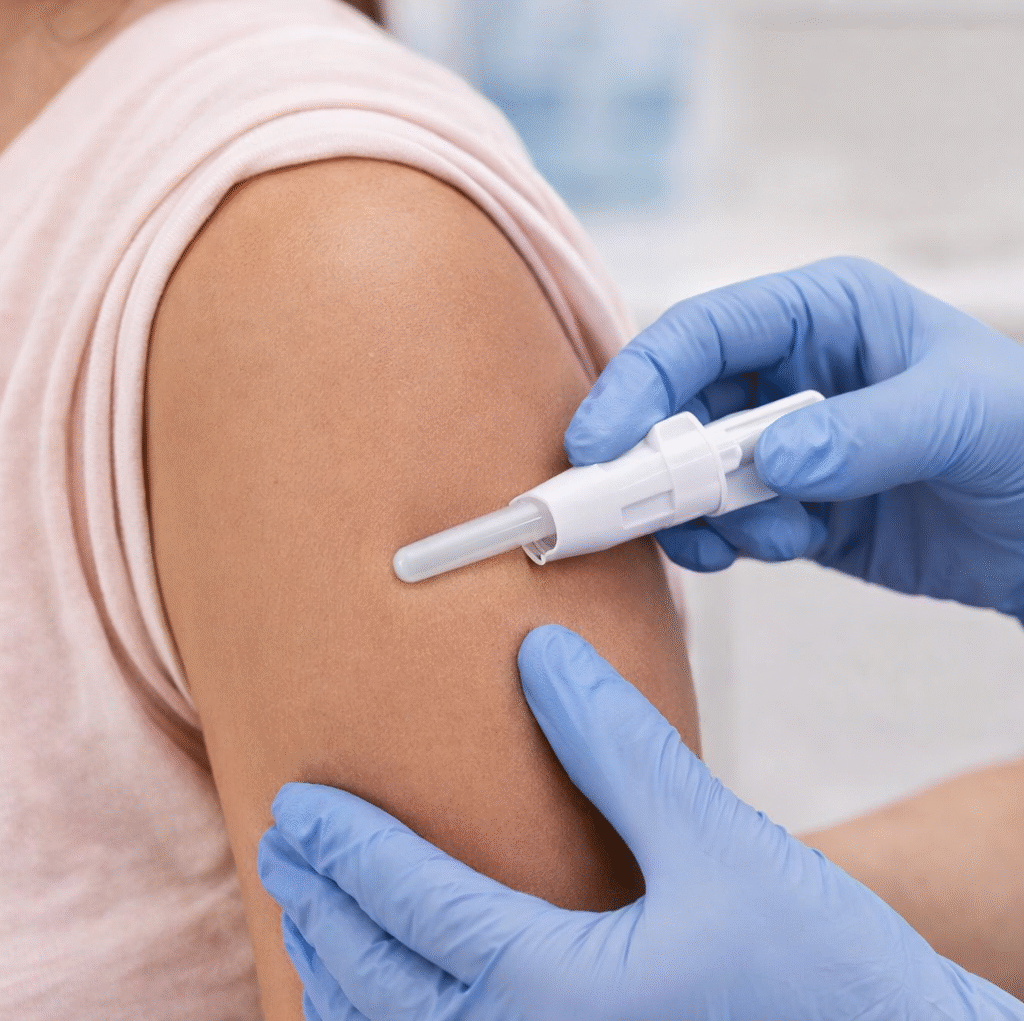
Organon’s NEXPLANON contraceptive implant receives FDA approval for use up to five years based on trial data showing zero pregnancies and no new safety concerns, alongside a new REMS program to support proper insertion and removal.
Written By: Nikita Chaudhari BPharm
Reviewed By: Pharmacally Editorial Team
Organon has received US Food and Drug Administration approval for a supplemental New Drug Application for NEXPLANON, the etonogestrel contraceptive implant. The decision extends the approved duration of use from three years to up to five years, giving women a longer period of highly effective pregnancy prevention without replacing the device.
Juan Camilo Arjona Ferreira, Organon’s Head of Research and Development, called the approval an important step for women’s health. He said the new data reflect Organon’s commitment to inclusive care and to providing long-acting options that meet the needs of real patients.
Trial Shows Strong Protection Through Year Five
The approval is based on a US multicenter open-label study (NCT04626596) that evaluated contraceptive efficacy during years four and five of use. Among 399 participants aged 18 to 35, investigators reported no pregnancies and no new safety concerns.
The calculated Pearl Index was 0.0 pregnancies per 100 women-years. The study included women with a wide range of body mass index values from 17.2 to 64.3 kg/m². More than one-third of participants had a BMI of 30 kg/m² or higher, supporting the effectiveness of the implant across diverse body types.
New REMS Program to Strengthen Safe Use
Along with the label update, the FDA has required a Risk Evaluation and Mitigation Strategy for NEXPLANON. The program aims to reduce complications related to improper insertion and removal of the implant.
Under the REMS, only certified healthcare providers will be able to insert or remove the device. Pharmacies and distributors must also register with the program and may supply NEXPLANON only to certified clinicians. The program will begin on February 23, 2026, and current providers will have six months to complete certification.
Dr. Anita Nelson, Professor of Obstetrics and Gynecology at Western University of Health Sciences, said the updated label mirrors the diversity of patients seen in everyday practice. She added that the REMS will reinforce high standards for insertion and removal and build further confidence in the method.
Key Safety Points
NEXPLANON remains available only through the REMS because improper placement can cause serious complications. Deep insertion may lead to nerve or vascular injury, and rare cases of migration to blood vessels or the lung have occurred. Providers must confirm that the implant is palpable immediately after placement and must localize any non-palpable device before removal.
The product should not be used in women with known or suspected pregnancy, a history of blood clots, liver tumors or active liver disease, unexplained vaginal bleeding, or known breast cancer. Women using the implant may experience changes in menstrual bleeding patterns, which represent the most common reason for discontinuation.
Other potential risks include ectopic pregnancy, thrombotic events, ovarian cysts, elevated blood pressure, and mood changes. The most frequent adverse reactions reported in trials were headache, vaginitis, weight gain, acne, breast pain, and abdominal pain.
Patients Implications
With the new approval, women can rely on a single NEXPLANON implant for up to five years of contraception. Fertility typically returns quickly after removal, and pregnancies have occurred as early as one to two weeks afterward.
NEXPLANON does not protect against HIV or other sexually transmitted infections. Patients should discuss individual risks and benefits with their healthcare provider and seek care if they cannot feel the implant in their arm.
References
Organon Announces US Food and Drug Administration Approval of Supplemental New Drug Application Extending Duration of Use of NEXPLANON® (etonogestrel implant) 68 mg Radiopaque, 16 January 2026, https://www.organon.com/news/organon-announces-us-food-and-drug-administration-approval-of-supplemental-new-drug-application-extending-duration-of-use-of-nexplanon-etonogestrel-implant-68-mg-radiopaque/
A Study to Assess Contraceptive Efficacy and Safety of Etonogestrel (ENG) Implant Beyond 3 Years of Use (MK-8415-060), ClinicalTrials.gov ID NCT04626596, https://clinicaltrials.gov/study/NCT04626596