Written By: Pharmacally Medical News Desk
Zai Lab has announced that China’s National Medical Products Administration (NMPA) has approved COBENFY (xanomeline and trospium chloride) for the treatment of schizophrenia, marking a major milestone as the first-in-class, non-dopaminergic therapy approved for this condition in China.
Commenting on the approval, Rafael G. Amado, President and Head of Global Research and Development at Zai Lab, said the decision represents a meaningful advance for schizophrenia care in China, where millions of adults continue to live with persistent symptoms or experience limiting side effects from existing treatments. He noted that COBENFY’s broad symptom control and differentiated safety profile could help reshape long-term disease management and address unmet patient needs.
Clinical Evidence Supporting Approval
The NMPA approval is supported by a comprehensive clinical package, including a Phase 1 pharmacokinetics study conducted in China, the Phase 3 China registration study ZL-2701-001, and data from three global EMERGENT clinical trials. Together, these studies demonstrated clinically meaningful improvements in schizophrenia symptoms with a safety profile distinct from traditional dopamine-based antipsychotics.
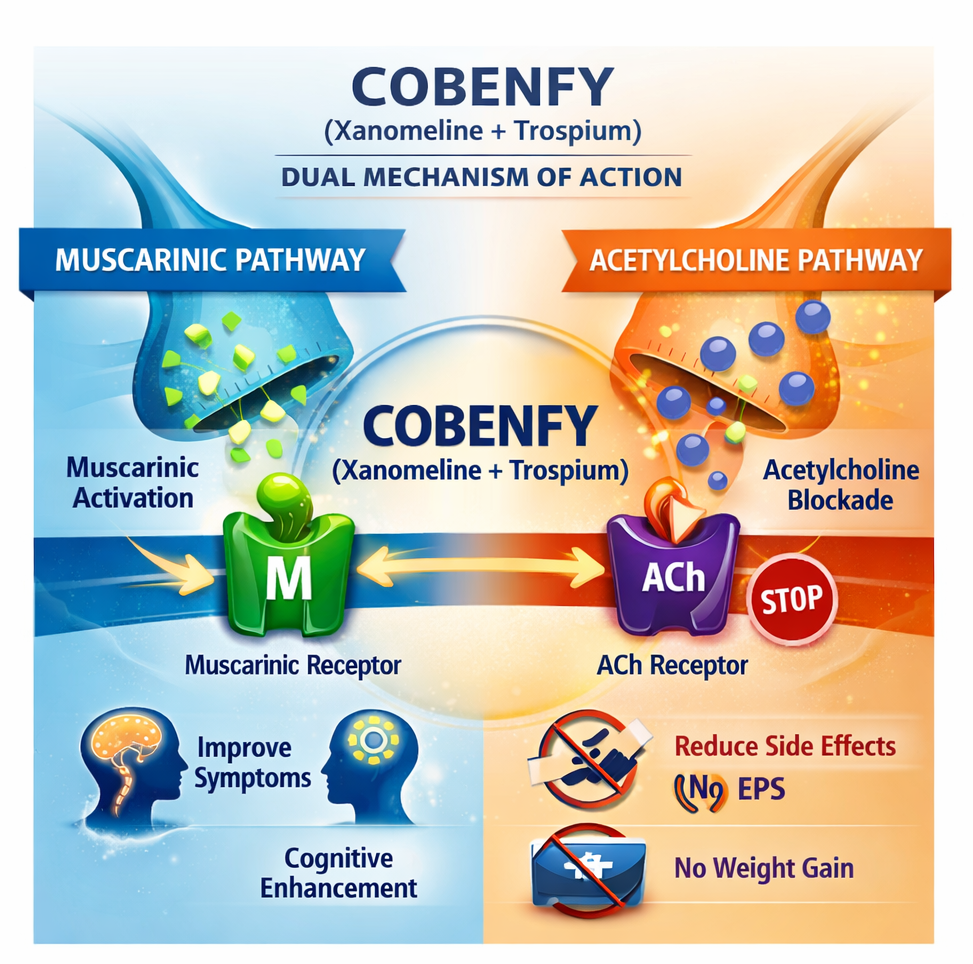
COBENFY combines xanomeline, a selective muscarinic receptor agonist, with trospium chloride, which limits peripheral cholinergic side effects. This mechanism allows central efficacy while reducing common tolerability issues seen with older therapies.
Following the clinical results and safety findings, Gang Wang, Dean of Beijing Anding Hospital at Capital Medical University and principal investigator of the Phase 3 China study, highlighted that COBENFY represents the first genuinely new therapeutic approach for schizophrenia in decades. He emphasized that the drug has shown improvement across positive, negative, and cognitive symptoms, while avoiding adverse effects such as weight gain, hyperprolactinemia, and extrapyramidal symptoms that often complicate long-term antipsychotic use.
Guideline Recognition in China
Notably, in September, the Chinese Medical Association released the China Schizophrenia Prevention and Treatment Guidelines (2025 Edition), which included COBENFY as a novel treatment option. This marks the first time a national-level guideline in China has formally recognized COBENFY, underscoring its clinical relevance even before regulatory approval.
With NMPA clearance now in place, Zai Lab plans to move swiftly toward making COBENFY available to patients in China, positioning the therapy as a new option for individuals who need effective symptom control with improved tolerability.
References
Zai Lab Announces Approval of COBENFY (xanomeline and trospium chloride) in China, a First-in-Class Therapy for Schizophrenia, 23 December 2025, https://ir.zailaboratory.com/news-releases/news-release-details/zai-lab-announces-approval-cobenfy-xanomeline-and-trospium

