Written By: Pharmacally Medical News Desk
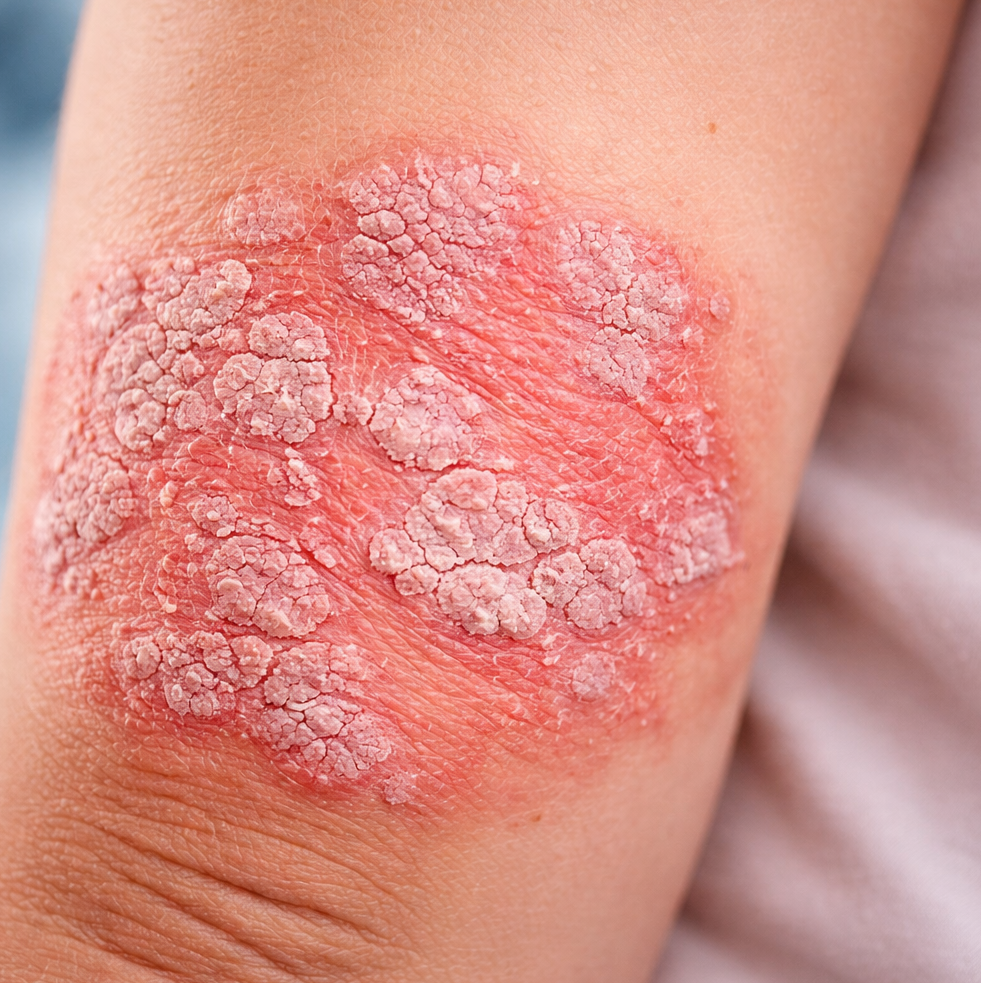
The European Commission has approved TREMFYA (guselkumab) for the treatment of children and adolescents with plaque psoriasis, marking the first pediatric indication for an IL-23 inhibitor in the European Union. This approval expands the use of TREMFYA beyond adults to younger patients with moderate to severe disease. The approval is supported by results from a pediatric Phase 3 clinical study evaluating guselkumab in children and adolescents with plaque psoriasis.
Marieke Seyger, Associate Professor, Radboud University Medical Centre Nijmegen, Netherlands, and PROTOSTAR study investigator, noted that treatment options for pediatric plaque psoriasis remain limited and the disease can significantly affect both physical and mental well-being during childhood. The approval of guselkumab provides physicians and caregivers with a new option that can meaningfully improve disease signs and symptoms in affected children.
Topline Results (PROTOSTAR Phase 3 Study)
The European Commission’s decision is supported by data from the Phase 3 PROTOSTAR study (EudraCT 2017-003053-42), which met both co-primary endpoints at Week 16 in pediatric patients with plaque psoriasis.
Key results from the Phase 3 PROTOSTAR study showed that 76% of pediatric patients treated with guselkumab achieved PASI 75, compared with 20% of those receiving placebo, indicating a significantly higher rate of at least 75% reduction in psoriasis severity with guselkumab. In addition, 66% of patients in the guselkumab group achieved an Investigator’s Global Assessment (IGA) score of 0/1, reflecting clear or almost clear skin, versus 16% in the placebo group, demonstrating that a majority of treated patients reached minimal or no visible disease by investigator assessment. Complete skin clearance (IGA 0) was observed in nearly 40% of patients receiving guselkumab, compared with 4% on placebo, showing that a substantial proportion of children achieved total clearance of skin lesions.
The safety profile of guselkumab subcutaneous injection in patients aged 6–17 years was consistent with that observed in adult plaque psoriasis studies. No new safety signals were identified in the pediatric population.
Mark Graham, Senior Director and Therapeutic Area Head for Immunology at Johnson & Johnson Innovative Medicine EMEA, said the approval of guselkumab as the first fully human IL-23 inhibitor for pediatric plaque psoriasis represents an important step for children and their caregivers and reaffirms the company’s commitment to ongoing research to support its long-term efficacy and safety in both pediatric and adult patients.
Guselkumab is a fully human monoclonal antibody that selectively binds to the p19 subunit of interleukin-23 (IL-23), a key cytokine driving immune-mediated inflammation. Its initial EU approval in November 2017 for moderate-to-severe plaque psoriasis in adults was supported by results from the Phase 3 VOYAGE 1 and VOYAGE 2 studies, which demonstrated significant skin clearance and a favorable safety profile compared with placebo and active comparator. The indication was expanded in November 2020 to include adults with active psoriatic arthritis who had an inadequate response or intolerance to prior disease-modifying antirheumatic drugs.
In 2025, the European Commission further approved guselkumab for ulcerative colitis and Crohn’s disease in adults, including intravenous and subcutaneous induction dosing followed by subcutaneous maintenance therapy.
Reference
European Commission approves TREMFYA® (guselkumab) for the treatment of children with plaque psoriasis, marking the first paediatric indication for an IL-23 inhibitor, 22 December 2025, https://www.jnj.com/media-center/press-releases/european-commission-approves-tremfya-guselkumab-for-the-treatment-of-children-with-plaque-psoriasis-marking-the-first-paediatric-indication-for-an-il-23-inhibitor#:~:text=BEERSE%2C%20Belgium%20(19%20December%202025,of%206%20years%20who%20are