Written By: Pharmacally Medical New Desk
Protagonist Therapeutics and Takeda presented longer-term 52-week data from the Phase 3 VERIFY study of Rusfertide at the 67th American Society of Hematology (ASH) Annual Meeting in Orlando, Florida, from December 6-9, 2025, demonstrating sustained hematocrit control and clinical response in patients with Polycythemia Vera (PV).
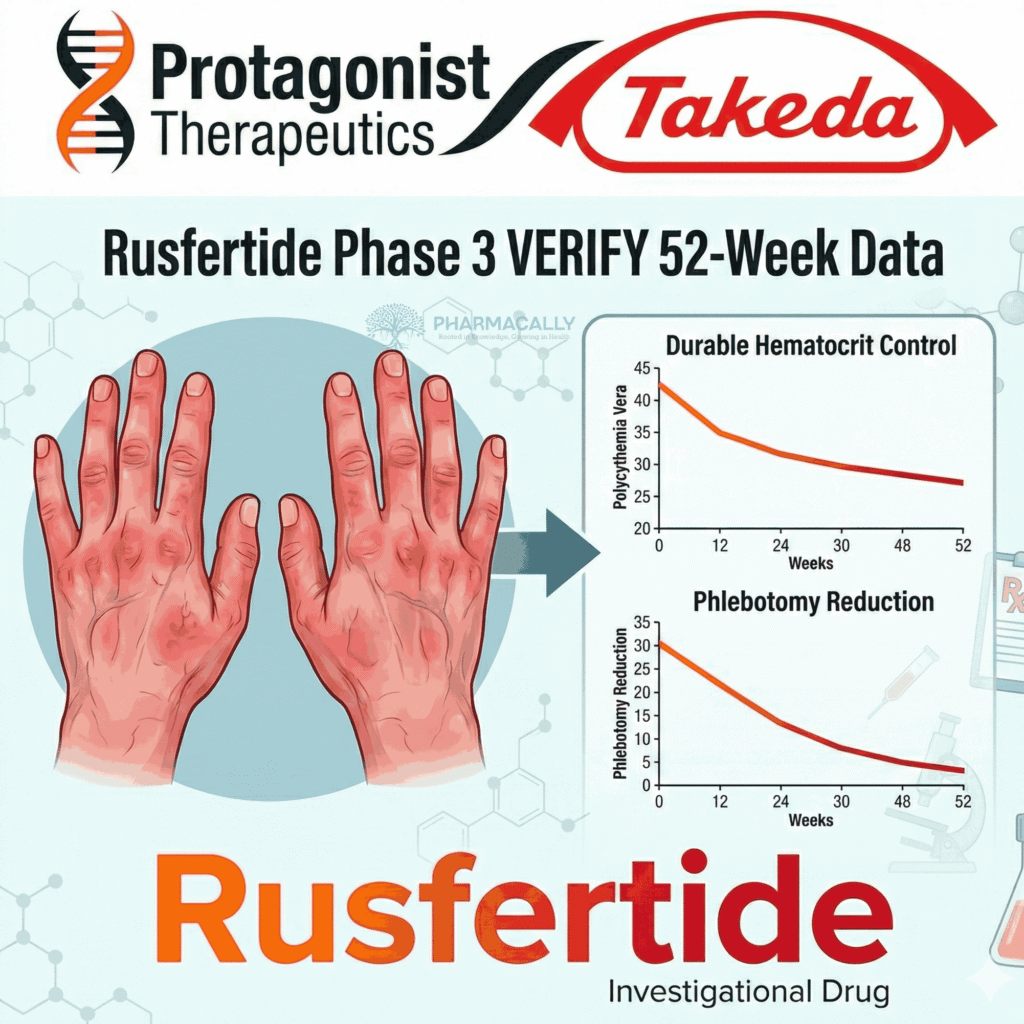
The investigational hepcidin mimetic peptide, administered subcutaneously once weekly, met its primary endpoint at 32 weeks and showed durability through week 52, with 61.9% of Rusfertide-treated patients maintaining absence of phlebotomy (blood removal) eligibility from baseline.
Dr. Andrew T. Kuykendall, VERIFY Lead Investigator at Moffitt Cancer Center, emphasized the significance of durable response data, stating that the 32-week VERIFY results were promising and that extended findings including THRIVE data reaffirm Rusfertide potential as a new standard of care for Polycythemia Vera patients.
Study Design and Key Efficacy Results
The VERIFY trial (NCT05210790) enrolled 293 PV patients randomized 1:1 to Rusfertide or placebo for 32 weeks (Part 1a), followed by open-label Rusfertide for all (Part 1b through week 52), with 91% completing the period and 254 entering long-term extension. Clinical response, defined as no phlebotomy eligibility (hematocrit ≥45% and ≥3% above baseline or ≥48%), was achieved by 61.9% of continuous Rusfertide patients from baseline to week 52, with 84.1% retaining response from weeks 20-32. Mean hematocrit stayed below 43% in Rusfertide arms, versus median time to ≥45% hematocrit of 8.3 weeks in placebo during Part 1a; placebo-to-Rusfertide switchers reached 77.9% response in weeks 40-52.
Long-term data from 46 patients transitioning from Phase 2 REVIVE (NCT04057040) to THRIVE (NCT06033586) showed a 13-fold reduction in annual phlebotomy rate versus baseline. Patient-reported outcomes improved with Rusfertide, including sustained benefits on fatigue and symptom burden as measured by PROMIS Fatigue SF-8a and the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score 7-item (MPN-SAF / MFSAF TSS7)
Safety Profile
Rusfertide was well-tolerated over 52 weeks, with common adverse events including injection site reactions (47%), anemia (25.6%), and fatigue (19.6%) mostly grade 1-2; serious adverse events occurred in 8.1%. No new safety signals emerged, consistent with prior observations.
Arturo Molina, Chief Medical Officer at Protagonist, highlighted Rusfertide’s positive impact on patients’ lives and expressed eagerness to collaborate with Takeda in preparing an NDA submission to the FDA.
Phuong Khanh (P.K.) Morrow, Head of Oncology Therapeutic Area Unit at Takeda, underscored the commitment to addressing the serious thrombotic risks faced by PV patients who cannot control hematocrit levels with current therapies, emphasizing rusfertide’s promise in fulfilling this critical unmet need.
About Rusfertide
Rusfertide (PTG-300) is an investigational, first-in-class hepcidin mimetic peptide. It binds to ferroportin, the iron-exporting protein on cells, leading to reduced iron release into the bloodstream. By lowering circulating iron and limiting iron delivery to the bone marrow, Rusfertide decreases erythropoiesis and helps many patients maintain hematocrit levels below 45%, reducing the need for therapeutic phlebotomy.
References
Protagonist and Takeda Present Longer-Term Data at ASH 2025 Showing Rusfertide Delivers Durable Response and Hematocrit Control in Polycythemia Vera, 06 December 2025, https://www.takedaoncology.com/newsroom/news-releases/2025/ash-rusfertide/
Protagonist and Takeda Present Longer-Term Data at ASH 2025 Showing Rusfertide Delivers Durable Response and Hematocrit Control in Polycythemia Vera, 06 December 2025, https://feeds.issuerdirect.com/news-release.html?newsid=7800905640169631&symbol=PTGX
A Phase 3 Study of Rusfertide in Patients with Polycythemia Vera (VERIFY), ClinicalTrials.gov ID NCT05210790, https://clinicaltrials.gov/study/NCT05210790
Hepcidin Mimetic in Patients with Polycythemia Vera (REVIVE), ClinicalTrials.gov ID NCT04057040, https://clinicaltrials.gov/study/NCT04057040
Study to Evaluate the Long-term Safety of Rusfertide (PTG-300) in Subjects with Polycythemia Vera (THRIVE), ClinicalTrials.gov ID NCT06033586, https://clinicaltrials.gov/study/NCT06033586