Written By: Pharmacally Medical News Desk
The FDA released the final “QTc Information in Human Prescription Drug and Biological Product Labeling Guidance for Industry” in December 2025. This document helps drug makers include clear QTc prolongation data in labels for non-antiarrhythmic drugs and biologics. It ensures prescribers and patients understand cardiac risks linked to QTc changes.
Scope of the Guidance
This guidance applies to:
Human prescription drug and biological products that are non-antiarrhythmic (i.e., not drugs whose primary indication is the treatment of arrhythmias) but which have systemic bioavailability
Products for which QTc-interval prolongation is relevant (either because of observed data or mechanistic risk)
Labeling changes for new approvals and updates to existing approved products.
It does not apply to antiarrhythmic drugs whose labeling already addresses QT/QTc risk in specialized ways.
What is QTc Prolongation?
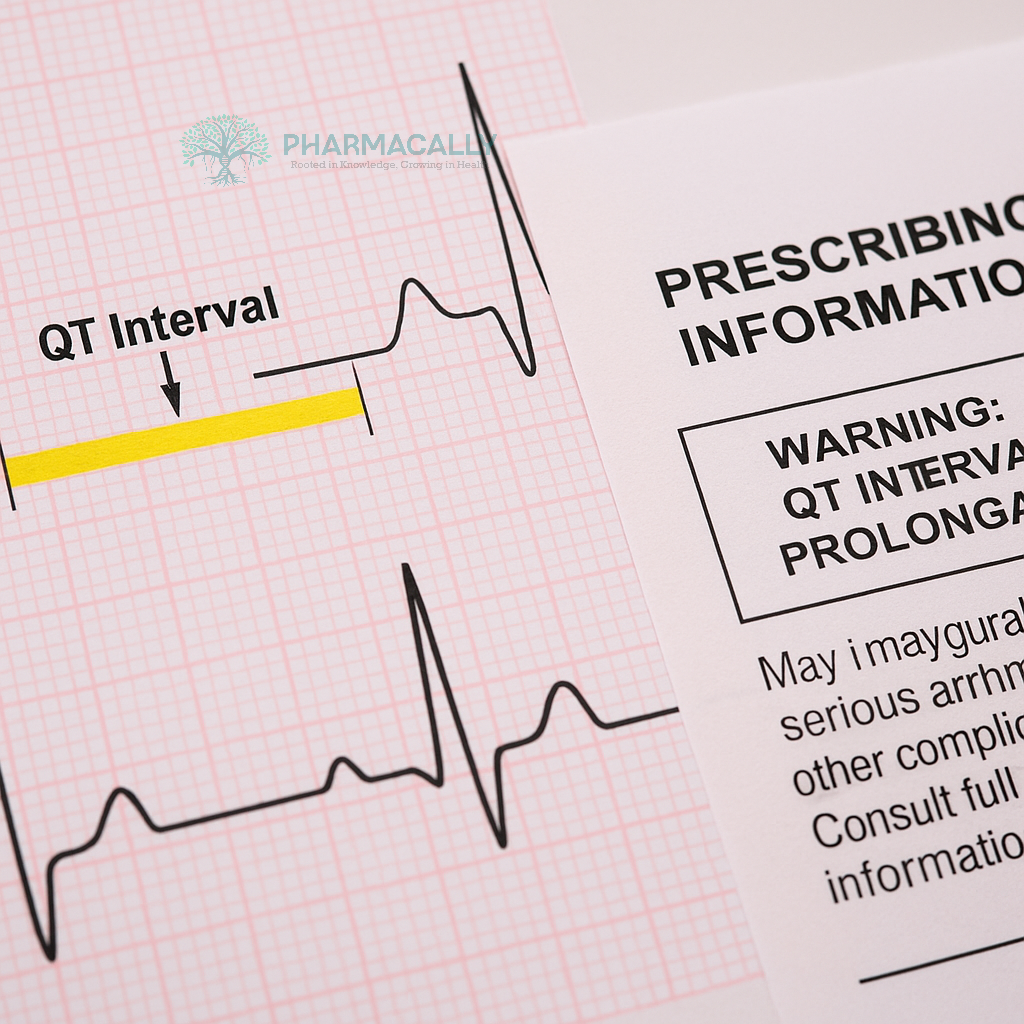
Drugs can delay heart repolarization, shown as a longer QT interval on an ECG, corrected for heart rate as QTc. This delay raises the risk of torsade de pointes (TdP), a dangerous rhythm that may lead to sudden death. Patients with factors like low potassium, heart failure, or other QT-prolonging drugs face higher risks.
The guidance builds on ICH E14 rules, which call for thorough QT (TQT) studies or alternatives like concentration-QTc modeling early in development. These tests check if a drug causes a mean QTc increase over 10 ms, a key concern threshold.
Clinical Pharmacology Section Details
Place QTc effect data under the “Cardiac Electrophysiology” heading in the Clinical Pharmacology section. Describe studied doses, exposure ranges, and any dose-response link to QTc changes. Use simple statements like: “At the maximum dose, no clinically significant QTc prolongation occurred,” if studies rule out a 10 ms mean increase.
For positive findings, state: “The largest mean QTc increase was X ms (upper CI Y ms) at [dose].” Omit this for drugs like monoclonal antibodies that skip QTc assessments.
Drug Interactions Coverage
List QTc-related interactions in a dedicated Drug Interactions subsection. Warn against combining with other QT-prolonging drugs, advising ECG checks if unavoidable. Explain risks like bigger QTc jumps leading to TdP.
For drugs where inhibitors boost levels and QTc effects, note: “Avoid strong CYP3A inhibitors as they raise drugozide-x exposure and QTc risks.” Always include mechanisms and management steps.
Warnings and Precautions Essentials
Include a warning if QTc prolongation links to serious events like TdP, ventricular tachycardia, or sudden death. Detail adverse reaction rates, like patients exceeding QTc >500 ms or >60 ms change from baseline. Advise baseline ECGs, electrolyte checks, and avoiding high-risk patients.
Mitigate risks by monitoring ECGs periodically, correcting electrolytes, and adjusting doses for QTc rises. Cross-reference Dosage and Administration for specifics.
Boxed Warning Requirements
FDA recommends a Boxed Warning for causal links to cardiac death, TdP, polymorphic ventricular tachycardia, or life-threatening arrhythmias with QTc prolongation. Place it at label start with trial or post-marketing evidence; cross-reference other sections for monitoring.
Dosage and Administration Modifications
Detail QTc-based dosage changes, holds, or stops in Dosage and Administration. Specify monitoring like ECGs every few weeks, based on pharmacokinetics and patient needs. Create subsections such as “2.x Dosage Modifications for QTc Interval Prolongation.”
Adverse Reaction Severity | Monitoring and Dosage Modifications |
Torsade de pointes, polymorphic ventricular tachycardia, or signs/symptoms of serious arrhythmia [see Warnings and Precautions (5.x)] | Permanently discontinue DRUG-X. |
QTc >500 ms absolute or >60 ms increase from baseline | Withhold until QTc <500 ms, resume at reduced dose. ECG every 2-4 weeks and as needed. Correct electrolytes. |
Merge with other adverse reaction tables if multiple risks apply. Tailor thresholds to data
Contraindications Guidance
Contradict use where QTc risks exceed benefits, like congenital long QT syndrome or uncompensated heart failure. Example: “DRUG-X contraindicates in congenital long QT or uncompensated heart failure [see Warnings and Precautions (5.x)].”
Ban combos with drugs causing unacceptable QTc spikes. Skip for life-threatening uses like cancer if monitoring allows benefit. Focus on clear harm without gain.
Adverse Reactions Reporting
List QTc events like TdP or arrhythmias with rates and contexts in Adverse Reactions. Match Warnings and Precautions for unity. Include percentages for QTc >500 ms or >60 ms changes over trial periods.
Note exclusion criteria like heart disease to frame data. Describe dose-dependent trends. This delivers raw safety stats separate from advice.
Patient Counseling Information
Summarize risks simply for patients: “DRUG-X prolongs QTc and raises TdP, arrhythmia, or sudden death risk. Expect ECGs before and during treatment. Seek urgent care for palpitations, dizziness, or fainting [see Warnings and Precautions (5.x)].”
Advice on interactions: “Tell your provider about all drugs, supplements. Avoid other QT-prolongers with DRUG-X.” Stress electrolyte importance and emergency signs.
Summary
The FDA’s “QTc guidance clarifies how drug and biologic sponsors should handle QTc-interval prolongation information in labeling. It stresses early assessment, clear placement of risk information in the prescribing information (including patient labeling), and ongoing updates when new data emerge. Someone working in medical writing, pharmacovigilance and health-information dissemination, this guidance offers rich content potential and ensures alignment with regulatory thinking when covering drug safety topics.
References
QTc Information in Human Prescription Drug and Biological Product Labeling Guidance for Industry, US Food and Drug Administration, December 2025, https://www.fda.gov/media/170814/download
QTc Information in Human Prescription Drug and Biological Product Labeling, 02 December 2025, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/qtc-information-human-prescription-drug-and-biological-product-labeling
QTc Information in Human Prescription Drug and Biological Product Labeling; Guidance for Industry; Availability, Federal Register, https://www.federalregister.gov/documents/2025/12/03/2025-21798/qtc-information-in-human-prescription-drug-and-biological-product-labeling-guidance-for-industry