Written By: Pharmacally Medical News Desk
Sarepta Therapeutics announced on 14 November 2025, that the U.S. Food and Drug Administration (FDA) has approved substantial updates to the prescribing information for ELEVIDYS (Delandistrogene Moxeparvovec-rokl), its gene therapy for Duchenne muscular dystrophy (DMD). These updates follow a comprehensive safety review triggered by reports of fatal acute liver failure in non-ambulatory pediatric patients receiving ELEVIDYS. The revised labeling implements critical safety measures aimed at protecting patients and guiding clinicians in optimal therapeutic use.
Key Updates in ELEVIDYS Labeling
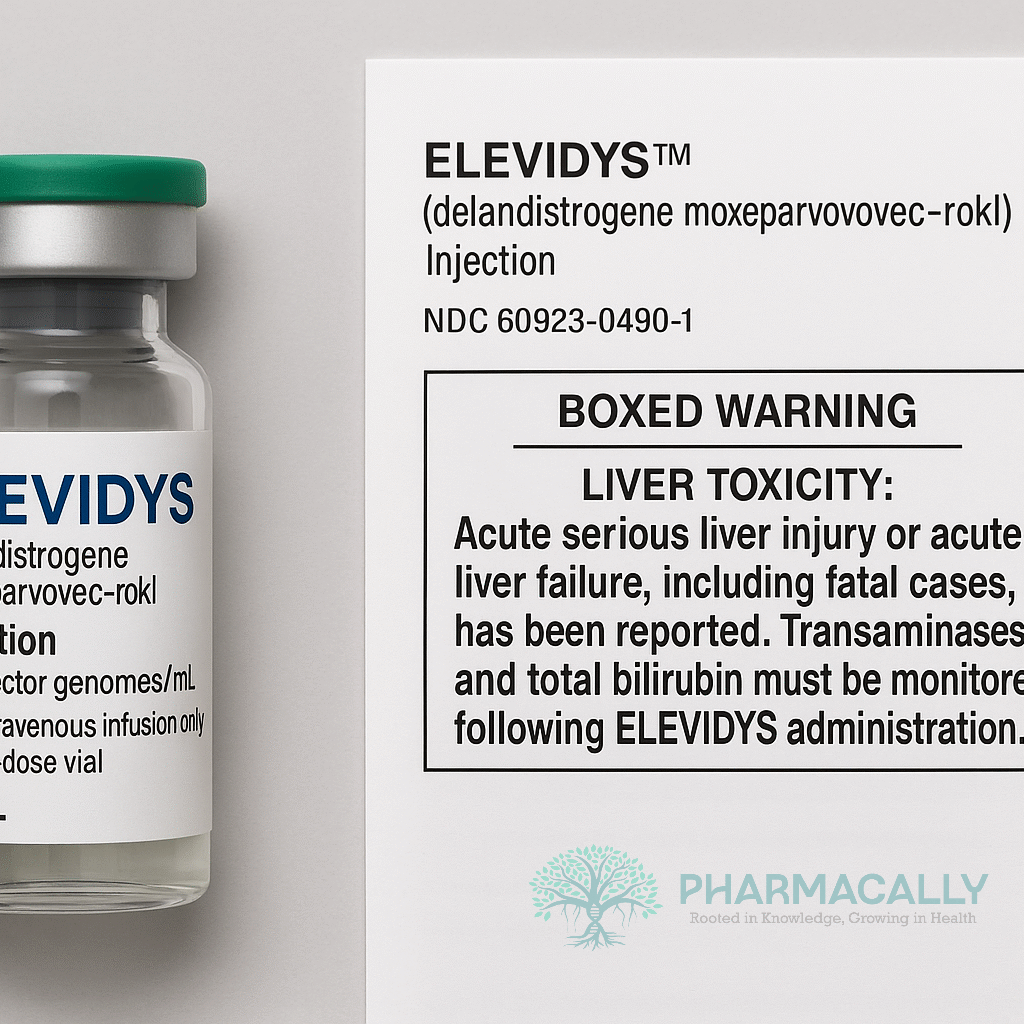
Boxed Warning Addition
The updated ELEVIDYS label now prominently features a Boxed Warning, the FDA’s strongest safety alert, highlighting the risk of serious liver injury and acute liver failure, including fatal outcomes. This warning reflects the FDA’s response to severe liver-related adverse events observed post-treatment.
Narrowed Patient Eligibility
The indication for ELEVIDYS has been significantly restricted. The therapy is now approved exclusively for ambulatory DMD patients aged four years and older with a confirmed mutation in the dystrophin gene. The label explicitly removes any indication for non-ambulatory patients, who were found to be at heightened risk of liver toxicity without demonstrable benefit. Additionally, ELEVIDYS is contraindicated in patients with deletions involving DMD exons 8 and/or 9.
Limitations of Use Statement
The update introduces a Limitations of Use section to guide physicians. It advises against ELEVIDYS use in patients with preexisting liver impairment, those recently vaccinated, or those with recent or active infections due to increased susceptibility to adverse effects.
Enhanced Safety Monitoring and Dosage Guidance
Revised labeling provides detailed recommendations for corticosteroid regimens before and after infusion and outlines rigorous monitoring protocols for the first three months post-administration. These measures are designed to detect and manage liver-related complications promptly.
Additional Warnings and Precautions
Immune-mediated myositis
Immune-mediated myositis is a serious muscle inflammation that can cause severe weakness, pain, and difficulty swallowing or breathing, dysphonia, and hypophonia. It occurs mainly in patients with specific deletions (exons 8 and/or 9) in the DMD gene and is why ELEVIDYS is contraindicated in these cases.
Myocarditis
Myocarditis is an inflammation of the heart muscle that can occur after ELEVIDYS treatment due to immune system activation. Though less common, it requires close monitoring to quickly address any cardiac symptoms and prevent serious complications.
Medication Guide Inclusion
A new Medication Guide for patients and caregivers accompanies the updated label, offering clear information on risks, symptoms to watch for, and the importance of follow-up care.
Post marketing Study Requirement
To further evaluate the safety profile, the FDA requires Sarepta to conduct a post marketing observational study involving approximately 200 DMD patients treated with ELEVIDYS. This study will include periodic liver function assessments for at least 12 months following therapy, aiming to better understand and mitigate long-term liver risks.
Key Monitoring Recommendations by FDA
The FDA’s updated ELEVIDYS labeling includes important safety information and recommendations for patients and healthcare providers to ensure close monitoring and prompt response to adverse effects:
Liver monitoring: Weekly liver function tests are recommended for at least three months after infusion. Patients should stay near a medical facility for at least two months post-treatment to manage potential liver complications.
Prompt medical attention: Patients must contact their healthcare provider immediately if they notice yellowing of the skin or eyes, miss or vomit corticosteroid doses, or experience changes in mental status.
Infection risk: Corticosteroid therapy may suppress the immune system, increasing vulnerability to infections, which can lead to severe complications including death.
Cardiac monitoring: Weekly cardiac injury testing (troponin-I) is advised for one month following treatment to detect immune-mediated cardiac effects early.
Clinical and Industry Impact
These FDA-mandated updates come nearly 18 months after ELEVIDYS attained full approval for DMD. They address critical safety concerns raised by healthcare providers and patient advocates, reinforcing a cautious but hopeful approach to gene therapy in this vulnerable population. Sarepta has indicated plans to study an enhanced immunosuppressive regimen for non-ambulatory patients in collaboration with the FDA, aiming to extend therapy options safely to this group.
Louise Rodino-Klapac, PhD, Sarepta’s President of R&D, acknowledged the FDA’s collaborative review process and emphasized the commitment to safety and informed clinical decision-making. The clarity provided by the updated prescribing information is expected to assist physicians and families in making better-informed treatment choices for DMD patients.
FDA leadership including CBER director Vinay Prasad during his brief tenure was publicly involved in decisions and communications about restricting Elevidys shipments amid the safety review. Prasad resigned from his FDA role in late July 2025 amid controversy over these and other decisions.
Basis of FDA Label Update: Liver Toxicity and Fatalities Linked to Sarepta’s AAVrh74 Vector Therapies
The FDA’s recent update to ELEVIDYS labeling and indication was driven by serious safety concerns arising in 2025 related to Sarepta’s AAVrh74 vector-based gene therapies. The first fatal liver toxicity case in a non-ambulatory Duchenne muscular dystrophy (DMD) patient occurred in March 2025, followed by a second death in June 2025. These acute liver failure events prompted the FDA to halt ELEVIDYS supply to non-ambulatory patients in June 2025. Additionally, in July 2025, a fatality was reported in a patient with limb-girdle muscular dystrophy treated with Sarepta’s SRP-9004 therapy, also using the AAVrh74 vector. These severe adverse events formed the basis for the FDA’s decision to add a Boxed Warning for liver injury and narrow ELEVIDYS’s indication exclusively to ambulatory DMD patients aged four and older to mitigate further risks.
References
Sarepta Announces FDA’s Approval of Updated ELEVIDYS Prescribing Information, Sarepta Therapeutics, 14 November 2025, https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-announces-fdas-approval-updated-elevidys-prescribing
FDA Approves New Safety Warning and Revised Indication that Limits Use for Elevidys Following Reports of Fatal Liver Injury, US FDA, 14 November 2025, https://www.fda.gov/news-events/press-announcements/fda-approves-new-safety-warning-and-revised-indication-limits-use-elevidys-following-reports-fatal
Highlights of prescribing information, ELEVIDYS (delandistrogene moxeparvovec-rokl) suspension, for intravenous infusion, https://cts.businesswire.com/ct/CT?id=smartlink&url=https%3A%2F%2Fwww.elevidys.com%2Fpi&esheet=54358487&newsitemid=20251114275201&lan=en-US&anchor=Prescribing&index=2&md5=9b76e2f0b69cdd8958223294377cce78
Medication Guide, ELEVIDYS, https://cts.businesswire.com/ct/CT?id=smartlink&url=https%3A%2F%2Fwww.elevidys.com%2Fmed-guide&esheet=54358487&newsitemid=20251114275201&lan=en-US&anchor=Medication+Guide&index=4&md5=5318deb965520ff3f3a12fb5083f2138
US FDA adds strongest warning to Sarepta’s Elevidys after fatal liver injuries, Reuters, 14 November 2025, https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-adds-strongest-warning-sarepta-gene-therapy-after-fatal-liver-injuries-2025-11-14/
After Second Liver Failure Death, Sarepta Therapeutics Halts Global Elevidys Dosing in Non-Ambulatory Duchenne Muscular Dystrophy Patients; Phase III ENVISION Trial Impacted, Pharmacally, 16 June 2025, https://pharmacally.com/sarepta-therapeutics-announced-second-death-from-liver-failure-of-its-duchenne-muscular-dystrophy-drug-elevidys-in-patients-who-are-unable-to-walk/
Fast Fade and Faster Return: What’s Behind Dr.Vinay Prasad’s Exit and Re-Entry at the FDA’s CBER? Pharmacally, 24 August 2025, https://pharmacally.com/fast-fade-and-faster-return-whats-behind-dr-vinay-prasads-exit-and-re-entry-at-the-fdas-cber/
FDA Halts Sarepta Therapeutics’ AAVrh74 based Gene Therapy Trials after Third Death from Liver Failure, 22 July 2025, Pharmacally, https://pharmacally.com/fda-halts-sarepta-therapeutics-aavrh74-based-gene-therapy-trials-after-third-death-from-liver-failure/