Written By: Pharmacally Medical News Desk
Bayer’s nonsteroidal mineralocorticoid receptor antagonist, finerenone (marketed as Kerendia™ and Firialta™), has demonstrated a statistically significant reduction in urine albumin-to-creatinine ratio (UACR) in adults with chronic kidney disease (CKD) associated with type 1 diabetes (T1D), according to results from the Phase 3 FINE-ONE clinical trial (NCT05901831).
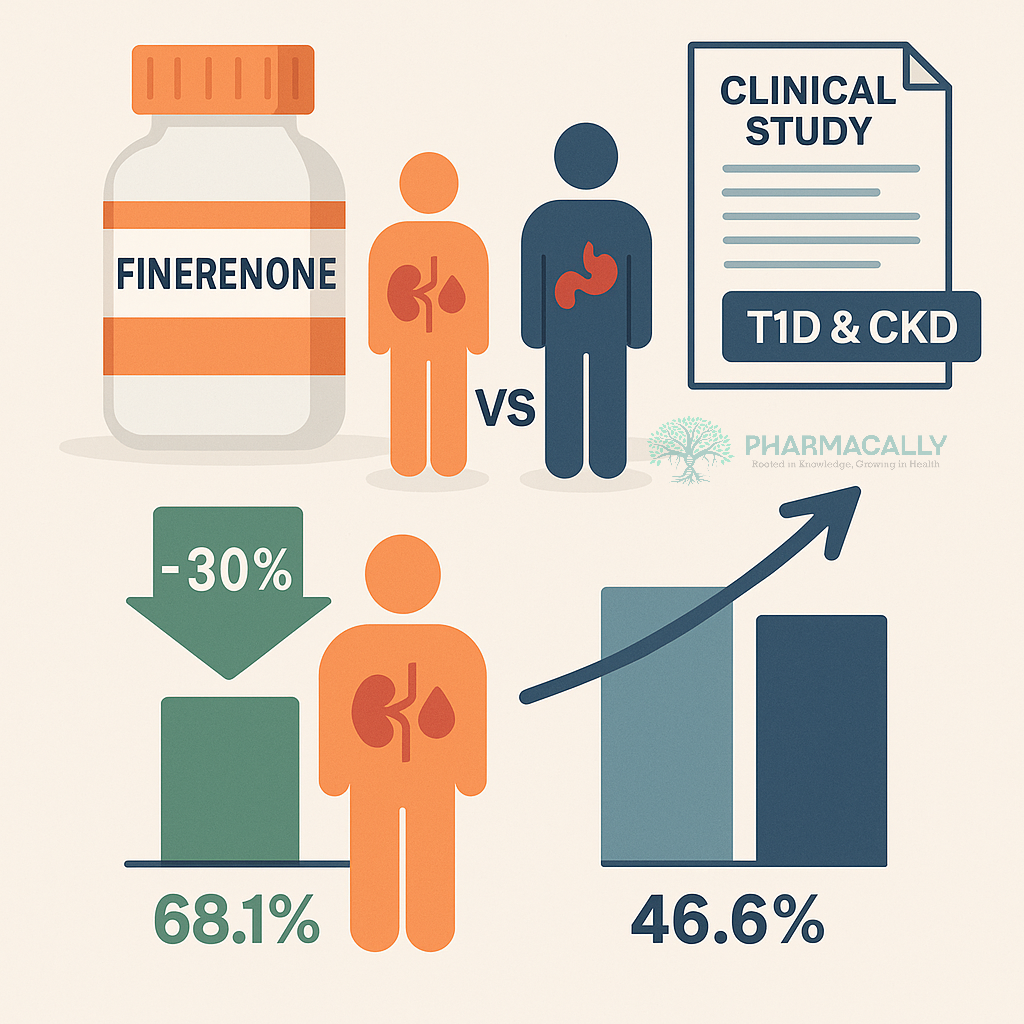
The study, led by Professor Hiddo Lambers Heerspink, Chair of the FINE-ONE Steering Committee, revealed that Finerenone achieved a notable improvement in kidney outcomes. At least a 30% reduction in UACR was seen in 68.1% of participants treated with Finerenone compared to 46.6% in the placebo group. The drug was well-tolerated, with few serious adverse events and a safety profile consistent with previous trials.
Finerenone acts by selectively blocking overactivation of the mineralocorticoid receptor, which plays a key role in inflammation and fibrosis leading to renal and cardiovascular damage. By mitigating the effects of aldosterone, Finerenone offers dual protection for both kidney and heart health.
“Patients with type 1 diabetes and CKD face a heightened risk of kidney failure and cardiovascular complications,” said Professor Heerspink. “The observed reduction in UACR correlates with slower disease progression and fewer adverse outcomes, signaling a major advancement for patients with limited treatment options.”
Supporting the clinical significance of these findings, Dr. Jonathan Rosen, Research Director at Breakthrough T1D, noted that kidney complications remain a critical unmet need in type 1 diabetes management. “These results strengthen the case for earlier and targeted intervention to preserve kidney function,” he said.
Dr. Christian Rommel, Global Head of Research and Development at Bayer Pharmaceuticals, emphasized the long-standing treatment gap in this area. “For more than three decades, patients with type 1 diabetes–related kidney disease had no disease-modifying therapy. Finerenone ability to meaningfully lower UACR redefines what’s possible in kidney protection,” he stated.
Building on the FINE-ONE results, Bayer plans to pursue regulatory discussions to expand Finerenone indications to CKD associated with T1D. Additionally, the company is preparing long-term extension and mechanistic studies to further explore how Finerenone impacts renal and cardiovascular outcomes over time, including potential combination regimens with SGLT2 inhibitors or GLP-1 receptor agonists.
If approved for this new indication, Finerenone could become the first therapy in over 30 years to provide meaningful kidney protection for individuals with T1D and CKD representing a milestone in diabetic kidney care.
References
Finerenone showed statistically significant reduction of UACR in adults with chronic kidney disease associated with type 1 diabetes, 06 Nov 2025, Bayer, https://www.bayer.com/media/en-us/finerenone-showed-statistically-significant-reduction-of-uacr-in-adults-with-chronic-kidney-disease-associated-with-type-1-diabetes/
A Study to Learn How Well the Study Treatment Finerenone Works and How Safe it is in People with Long-term Decrease in the Kidneys’ Ability to Work Properly (Chronic Kidney Disease) Together With Type 1 Diabetes (FINE-ONE), ClinicalTrials.gov ID NCT05901831, https://clinicaltrials.gov/study/NCT05901831
Heerspink HJL et al, Rationale and design of a randomised phase III registration trial investigating finerenone in participants with type 1 diabetes and chronic kidney disease: The FINE-ONE trial. Diabetes Res Clin Pract. 2023 Oct; 204:110908. doi: 10.1016/j.diabres.2023.110908. Epub 2023 Oct 5. PMID: 37805000.