Written By: Arbaz Saiyyad, DPharm, BSc, BPharm
Reviewed By: Pharmacally Editorial Team
The U.S. Food and Drug Administration (FDA) has approved Lasix ONYU (furosemide 80 mg/2.67 mL oral suspension) as a novel, subcutaneous, drug-device combination for treating edema associated with chronic heart failure, liver cirrhosis, and renal disease.
The development and commercialization of Lasix ONYU involved a multi-company collaboration. SQ Innovation, Inc is the primary developer and Marketing Authorization Holder of Lasix ONYU. SQ Innovation designed the complete Lasix ONYU therapy model including clinical protocols to enable subcutaneous administration at home. Gerresheimer, a German-based medical device engineering company, was responsible for designing, developing, and manufacturing the ONYU Infusor system. Ligand Pharmaceuticals supplied the Captisol® technology, a proprietary cyclodextrin platform that enhances aqueous solubility, bioavailability, and stability of furosemide at high concentration (30 mg/mL).
About Lasix ONYU and how it works
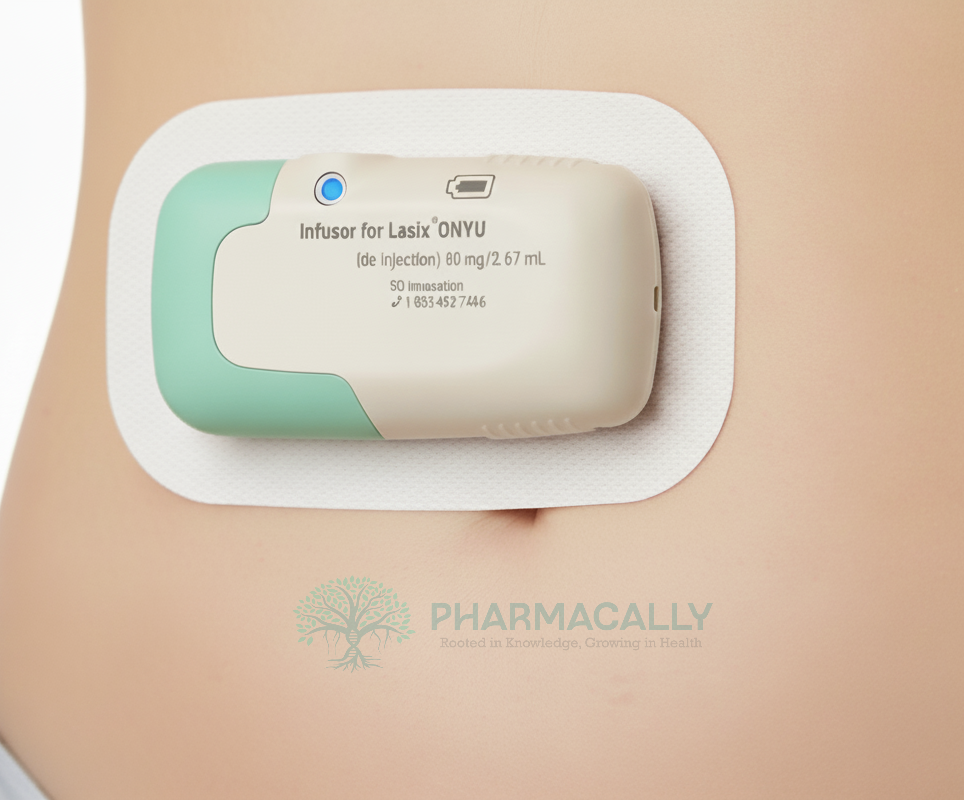
Lasix ONYU integrates a high-concentration formulation of furosemide (30 mg/mL) with an on-body Infusor that enables subcutaneous (under-the-skin) infusion at home.
The drug is delivered via the Lasix ONYU Infusor, a small, wearable, two-part device that attaches to the patient’s abdomen. The disposable unit holds an 80 mg (2.67 mL) furosemide glass cartridge and a 29-gauge needle, which inserts under the skin upon activation.
This biphasic slow delivery leads to steady therapeutic plasma concentrations of furosemide within 30 minutes that are maintained across the infusion period, avoiding the rapid peak and drop seen with IV bolus injections.
The reusable electromechanical unit controls the infusion pump, delivering the full dose over approximately five hours at a constant, controlled rate, mimicking a slow intravenous infusion but without the need for a catheter or hospital stay.
This design eliminates the need for intravenous administration and offers portability and convenience for patients. Ligand’s Captisol® technology ensures solubility and stability of the furosemide formulation while reducing irritation at the infusion site.
About the Indications
FDA approval covers Lasix ONYU for managing edema resulting from heart failure, liver cirrhosis, and renal disease. The drug aims to treat patients with fluid overload outside the hospital setting. Heart failure currently affects around 6.7 million Americans, a number expected to rise to 8.7 million by 2030, making accessible home-based diuretic therapies a priority for the healthcare system.
Clinical Evidence
Clinical studies related to bioavailability and diuretic response conducted by SQ Innovation and University of Glasgow teams, demonstrated complete bioavailability (112%) compared with IV furosemide bolus administration, and equivalent diuresis (115%) and natriuresis (117%) levels. Lasix ONYU’s controlled, prolonged delivery mitigated the rapid, high-peak diuretic effect seen with IV bolus, providing a more balanced fluid removal response. These data confirmed non-inferiority at the same time offering improved tolerability and patient comfort.
Safety Profile
The most common adverse effects included mild local site reactions such as erythema or pruritus. Systemic side effects paralleled those of conventional furosemide therapy, including electrolyte imbalance and dehydration risk. The subcutaneous administration route also reduced the frequency of hospitalization-related infections and adverse events linked to IV catheterization.
Implications for Patients
Lasix ONYU represents a major advance for patient care toward home-based chronic disease care. It allows selected patients to avoid repeated hospital admissions for intravenous diuresis, thereby reducing healthcare costs and improving quality of life. The reusable component minimizes waste and treatment costs, with the design supporting patient autonomy and remote monitoring integration for physicians.
Expert and Official Opinions
Dr. Pieter Muntendam, CEO of SQ Innovation, described Lasix ONYU as “transformative for patients experiencing worsening heart failure due to fluid overload,” emphasizing its potential to unburden hospitals. Dr. Javed Butler, President of Baylor Scott & White Research Institute, stated that treating more patients at home with subcutaneous diuretic technology “addresses the growing resource strain in cardiovascular care”. Industry executives from Ligand and Gerresheimer noted that the approval validates their integrated device-formulation approach, demonstrating how partnerships can accelerate innovation in chronic disease management.
The FDA approval of Lasix ONYU marks a milestone in digital-enabled, home-based cardiology care. By combining advanced drug delivery engineering with proven diuretic pharmacology, SQ Innovation’s therapy offers both clinical equivalence and logistical convenience. The anticipated rollout of ONYU is in Q4 2025 aligns with growing policy emphasis on home-treatment modalities, reshaping the management landscape for patients with chronic edema due to heart, liver, or renal disease.
References
SQ Innovation Announces FDA Approval of Lasix® ONYU for Treatment of Edema in Heart Failure, 08 Oct 2025, https://sqinnovation.com/fda-approval/
Gerresheimer: FDA grants Approval of SQ Innovation’s Lasix® ONYU*, Gerresheimer, 13 Oct 2025, https://www.gerresheimer.com/en/customer/company/news/detail/gerresheimer-fda-grants-approval-of-sq-innovations-lasixr-onyu
Ligand Partner SQ Innovation Receives FDA Approval for Lasix® ONYU, an At-Home Treatment for Edema in Heart Failure Patients, Ligand, https://investor.ligand.com/news-and-events/press-releases/news-details/2025/Ligand-Partner-SQ-Innovation-Receives-FDA-Approval-for-Lasix-ONYU-an-At-Home-Treatment-for-Edema-in-Heart-Failure-Patients/default.aspx