Written By: Arbaj Saiyyad BPharm
Reviewed By: Pharmacally Editorial Team
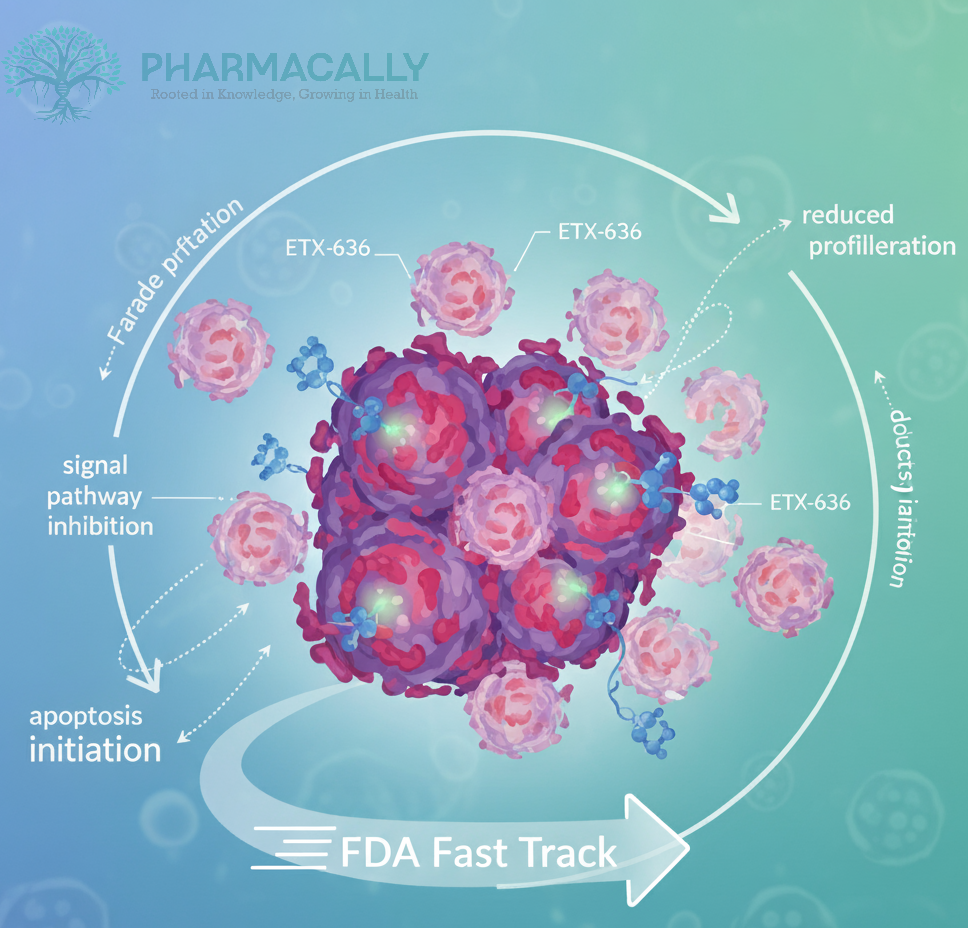
Ensem Therapeutics has announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to its investigational drug ETX-636 for the treatment of PIK3CA-mutant, hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer. The designation, confirmed in early October 2025, highlights the potential of ETX-636 to address an unmet need in patients with advanced breast cancer who have limited targeted treatment options.
About ETX-636
ETX-636 is an experimental, oral small molecule designed to selectively target and degrade mutant forms of the PI3Kα enzyme, which are commonly seen in certain breast cancers. It was developed using Ensem’s Kinetic Ensemble® platform, which combines artificial intelligence and computational modeling to create drugs that bind precisely to specific sites on target proteins.
According to the company, ETX-636 works as a pan mutant-selective allosteric inhibitor and degrader of PI3Kα. This means it is designed to block and promote the breakdown of the mutant PI3Kα protein while sparing the normal (wild-type) version. The goal of this selective mechanism is to limit side effects such as hyperglycemia (high blood sugar), which are often associated with older, non-selective PI3K inhibitors.
While these findings are based on preclinical research, the approach represents a promising attempt to improve both the safety and precision of PI3K-targeted cancer therapies.
About PIK3CA Mutations and Breast Cancer
Mutations in the PIK3CA gene activate the PI3K pathway, a key driver of tumor growth and survival. These mutations occur in about 40% of HR+/HER2- breast cancers, a subtype that accounts for roughly 70% of all breast cancer cases. Patients with such mutations often face resistance to standard hormonal therapies, making the search for safer and more effective targeted treatments especially important.
Current Clinical Trial
ETX-636 is currently being evaluated in an ongoing Phase 1/2 first-in-human clinical trial (NCT06993844). The study is assessing the drug’s safety, tolerability, pharmacokinetics, and early signs of antitumor activity, both as a monotherapy and in combination with fulvestrant, a selective estrogen receptor degrader already approved for HR+/HER2- breast cancer.
As of now, no clinical efficacy data have been released, and the investigational status of ETX-636 means its benefit and safety in patients remain to be established.
Significance of Fast Track Designation
The FDA’s Fast Track program is designed to speed up the development and review of drugs that may address serious or life-threatening conditions with limited treatment options. This status allows for closer communication with the FDA, rolling submission of data, and eligibility for priority review if results are favorable.
It’s important to note that Fast Track status does not mean the drug is approved or proven effective. Instead, it recognizes that early data suggest potential benefit worth expedited evaluation.
Ensem Therapeutics and Its Platform
Ensem Therapeutics is a clinical-stage biotechnology company focused on precision oncology. Its Kinetic Ensemble® platform integrates AI, deep learning, and biophysical modeling to design small molecules for difficult-to-target proteins involved in cancer and other diseases. ETX-636 is one of the first clinical candidates to emerge from this technology, representing Ensem’s efforts to translate computational design into real-world cancer therapeutics.
Public Health Implications
If clinical studies confirm its safety and efficacy, ETX-636 could offer a new targeted treatment for patients with PIK3CA-mutant HR+/HER2- breast cancer a group with limited therapeutic options after progression on standard therapies.
However, further human data are needed to validate the preclinical promise of this drug, especially regarding its mutant selectivity, reduced metabolic side effects, and overall clinical benefit.
References
Ensem Therapeutics Announces ETX-636 Granted Fast Track Designation by the FDA for Advanced Breast Cancer, Businesswire, 01 Oct 2025, https://www.businesswire.com/news/home/20251001083267/en/Ensem-Therapeutics-Announces-ETX-636-Granted-Fast-Track-Designation-by-the-FDA-for-Advanced-Breast-Cancer
Phase 1/2 Study of ETX-636 in Participants with Advanced Solid Tumors, ClinicalTrials.gov ID NCT06993844, https://www.clinicaltrials.gov/study/NCT06993844
The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012), https://doi.org/10.1038/nature11412
Goel, S. et al., PIK3CA mutations in HER2-positive breast cancer: an ongoing conundrum, Annals of Oncology, Volume 27, Issue 8, 1368 – 1372