Written and Review By: Pharmacally Medical News Desk
In recent development the U.S. Food and Drug Administration (FDA) approved Wayrilz (rilzabrutinib) tablets for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment. The marketing authorization holder is Sanofi. This approval makes Wayrilz the first Bruton’s tyrosine kinase (BTK) inhibitor approved in the United States for ITP.
This approval is meant for adults living with persistent or chronic ITP who have not achieved adequate benefit from earlier therapies. Many patients with ITP relapse or remain dependent on steroids, intravenous immunoglobulin (IVIG), or thrombopoietin receptor agonists (TPO-RAs), other patient including who failed therapies such as rituximab or even splenectomy. Wayrilz offers these patients a new treatment approach.
About Wayrilz
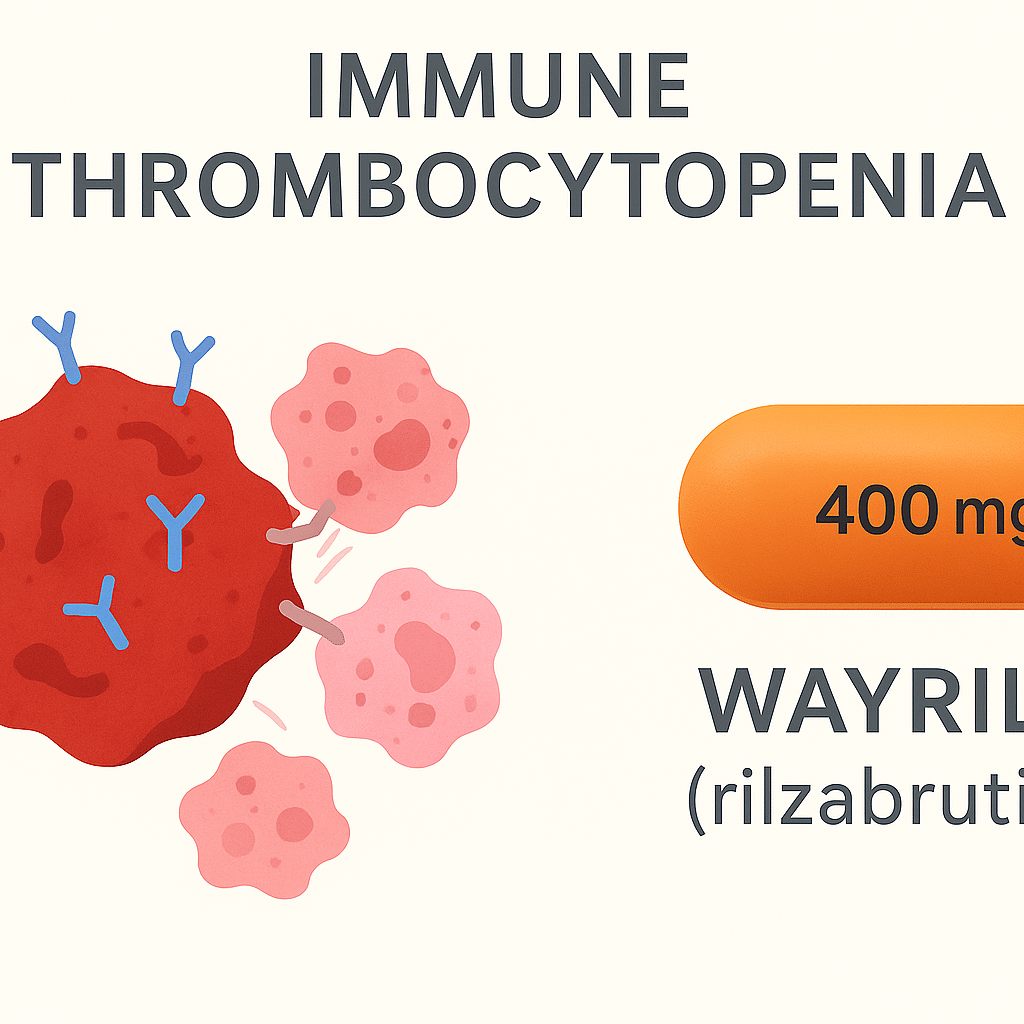
Wayrilz contains the active substance rilzabrutinib, a covalent but reversible inhibitor of Bruton’s tyrosine kinase (BTK). It is supplied as an orange, capsule-shaped, film-coated tablet of 400 mg strength. The recommended dose is 400 mg taken twice daily, with or without food.
In immune thrombocytopenia, the immune system produces antibodies that mark platelets for destruction. These antibodies also activate B cells, which sustain the autoimmune reaction. BTK is an important enzyme in both B cells and immune cells that engulf antibody-coated platelets. By blocking BTK, rilzabrutinib reduces B-cell activation and antibody production, while also preventing platelets from being destroyed in the spleen and liver. Although the drug leaves the bloodstream quickly, its binding to BTK is long-lasting, allowing sustained effect with twice-daily dosing.
Wayrilz provides an oral, first-in-class option that works differently from currently available therapies. It has shown the ability to produce durable platelet responses, reduce the need for rescue therapies, and achieve platelet recovery within about five weeks on average. For patients who have cycled through multiple therapies, this offers a meaningful alternative.
Wayrilz is indicated for adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment
Clinical Trial Evidence
The approval is based on results from the phase 3 LUNA-3 trial (NCT04562766). This was a randomized, double-blind, placebo-controlled study involving 202 adults with persistent or chronic ITP who had received multiple prior treatments. Participants were randomized in a 2:1 ratio to receive either Wayrilz 400 mg twice daily or placebo for 24 weeks. Background ITP therapies such as corticosteroids and TPO-RAs were allowed if doses were stable.
Key results
- Durable platelet response was achieved by 23% of patients on Wayrilz compared with none on placebo.
- The average number of weeks with platelet counts above 50×10⁹/L was 7.2 weeks with Wayrilz versus less than 1 week with placebo.
- The median time to first platelet response was 36 days with Wayrilz, while it was not reached in the placebo group.
- The need for rescue therapy was significantly reduced: 33% in the Wayrilz arm versus 58% with placebo.
These findings confirm that Wayrilz improves platelet counts and reduces disease flares compared to placebo
Safety Profile
The most common side effects reported with Wayrilz were diarrhea, nausea, abdominal pain, headache, joint pain, and infections such as COVID-19. Most were mild to moderate in intensity. One case of fatal pneumonia was reported in the trial.
Warnings and precautions include:
- Risk of serious infections: patients should be monitored and treated promptly.
- Liver toxicity, including drug-induced liver injury: regular liver tests are required.
- Potential harm to the fetus: pregnancy must be avoided during treatment and for one week after the last dose. Breastfeeding is not recommended during and for one week after treatment.
Wayrilz should not be used in patients with moderate to severe liver impairment or severe kidney impairment. It also interacts with certain medications, including strong CYP3A inhibitors and inducers. Acid-reducing agents such as proton pump inhibitors should be avoided as they lower drug levels significantly. Patients should also avoid grapefruit, starfruit, and Seville oranges during treatment.
Public Health Impact
Immune thrombocytopenia remains a challenging condition when first-line treatments fail. Patients often require repeated rescue therapies and long-term steroid exposure, which carries significant risks. The approval of Wayrilz represents a major step forward by offering a new mechanism of action and a convenient oral option that can achieve durable platelet responses. Its availability is expected to expand choices for both physicians and patients, improving long-term disease management.
Conclusion
The FDA approval of Wayrilz (rilzabrutinib) tablets provides a much-needed new option for adults with persistent or chronic ITP who have not responded to prior treatments. With evidence of durable platelet responses, reduced reliance on rescue therapies, and an overall manageable safety profile, Wayrilz adds significant value to the current treatment landscape. This first-in-class BTK inhibitor represents a new chapter in ITP management.
References
U.S. Prescribing Information – WAYRILZ™ (rilzabrutinib), Sanofi, August 2025 https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/219685s000lbl.pdf
Press Release: Sanofi’s Wayrilz approved in US as first BTK inhibitor for immune thrombocytopenia, Sanofi, 29 Aug 2025, https://www.sanofi.com/en/media-room/press-releases/2025/2025-08-29-21-50-18-3141825
David J. Kuter, Waleed Ghanima, Nichola Cooper et al, Safety and efficacy of rilzabrutinib vs placebo in adults with immune thrombocytopenia: the phase 3 LUNA3 study. Blood 2025; 145 (24): 2914–2926. Doi: https://doi.org/10.1182/blood.2024027336
Kuter DJ, Bussel JB, Ghanima W, et al, Rilzabrutinib versus placebo in adults and adolescents with persistent or chronic immune thrombocytopenia: LUNA 3 phase III study. Ther Adv Hematol. 2023 Oct 18; 14:20406207231205431. Doi: 10.1177/20406207231205431. PMID: 37869360; PMCID: PMC10585997.