By Team Pharmacally
Article Updated On: 07 May 2025
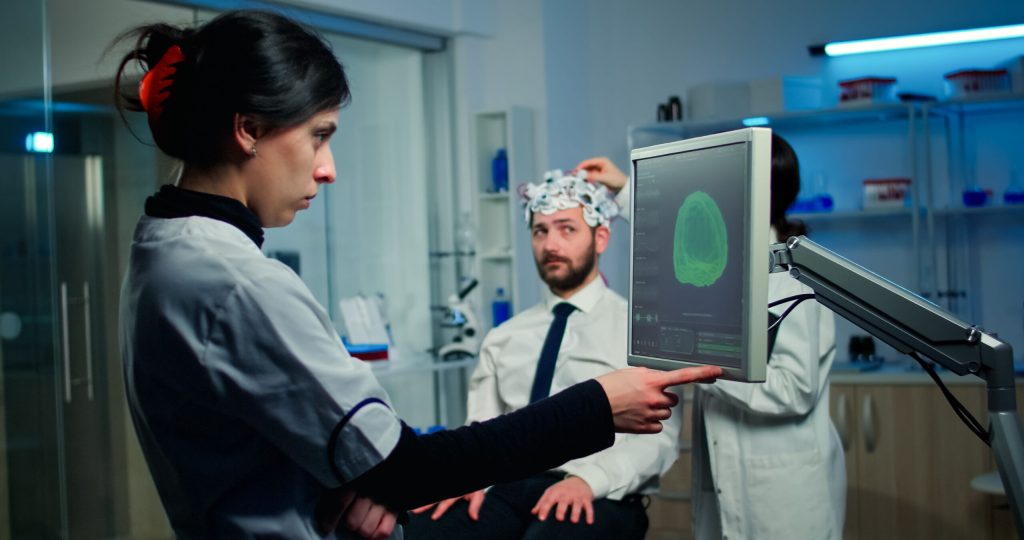
Neuralink, founded by Elon Musk, the neurotechnology company has taken great steps in getting FDA-certified brain implants, mainly in aiding individuals with paralysis and vision impairment.
Advancements in Assisting Paralysis
In January 2024, Neuralink implanted its N1 brain computer interface (BCI) chip into quadriplegic patient Noland Arbaugh. The implant allowed Arbaugh to control a computer cursor with his thoughts, marking a significant milestone in BCI technology. However, the device encountered some problems, such as the detachment of some electrodes, which affected its performance. Neuralink addressed these issues by refining their algorithms and surgical techniques. Then, another patient received a better implant that showed excellent stability and function. This patient was able to play video games and design 3D objects with computer-aided design software by August 2024.
Initiatives in Vision Restoration
In September 2024, the “Blindsight” implant from Neuralink received a so-called “breakthrough device” designation by the FDA. The implant is designed to restore vision for people who have both lost their eyes and their optic nerve. Optimistically, this may even benefit people born blind. According to the FDA, breakthrough devices are medical devices that provide a more effective treatment or diagnosis for a life-threatening or irreversibly debilitating condition. Although this acceptance speeds up the development process, it does not mean that the market will approve it right away. Neuralink has not yet set a date for human trials of the Blindsight implant.
Regulatory Milestones and Future Directions
In May 2023, Neuralink received approval from the FDA to start conducting human clinical trials. The early tests have involved individuals with serious spinal cord injuries in order to determine the safety and effectiveness of the implants. The owner of the corporation, Elon Musk, has stated that he would want to increase the size of those participating in the experiments and that, by the end of 2025; devices will be implanted in 20 to 30 other people. These developments highlight Neuralink’s dedication to enhancing BCI technology for the improvement of life quality for those with neurological impairments.
In summary, Neuralink FDA-approved brain implants are reportedly showing much promise in early human trials, especially in restoring motor functions for paralyzed individuals. In addition to the ongoing vision restoration, the BCI technology is only expected to revolutionize the field to deal with other various neurological challenges.
Update on 07 May 2025 :
Neuralink Patient Edits YouTube Video Using Only His Mind
Brad Smith, a dad from Arizona who has ALS (amyotrophic lateral sclerosis), is the third person in the world and the first who can’t speak to get a brain chip from Neuralink. This chip lets him control a computer using just his thoughts, and he was able to make and narrate a YouTube video using only his brain.
The Neuralink chip was placed in the part of Brad Smith’s brain that controls movement. It lets him move a computer mouse by just thinking about certain actions, like moving his tongue or clenching his jaw. This worked better than trying to imagine moving his hands. The chip connects wirelessly to a MacBook, turning his thoughts into actions on the screen.
To help him speak again, Brad used artificial intelligence that was trained on recordings of his voice from before he got ALS. This AI recreated his voice, allowing him to narrate his video. It’s an important step forward in technology that helps people communicate.
Before getting the Neuralink chip, Brad used eye-tracking technology to communicate, but it only worked well in certain lighting. Now, with the brain chip, he can communicate more easily in different places, even outside. He can also do fun things like play video games with his kids, which has made his life better.
Brad’s story shows how brain chips like Neuralink can help people with serious physical challenges regain control and communicate again. His experience gives hope that this kind of technology can make life better for others with similar conditions in the future.
Video Source: Mr. Smith Gets a Neuralink Brain Implant and a Visit From Elon Musk – Neuralink (YouTube)
Disclaimer: “This video is shared from Neuralink’s official YouTube channel and used here for informational and educational purposes.”
References:
1. Neuralink implanted second trial patient with brain chip, Musk says, Reuters, 5 Aug 2024
2. Neuralink’s first brain chip implant developed a problem — but there was a workaround, CNN Business, 09 May 2024
3. Neuralink’s Brain Chip: How It Works and What It Means, Capitol Technology University, 09 Feb 2024
4. Waisberg, Ethan; Ong, Joshua1; Lee, Andrew G.2, 3,4,5,6,7,8,9. The potential to restore vision with Neuralink’s “Blindsight” neural interface technology. The Pan-American Journal of Ophthalmology 6(3):84, May 2024. | DOI: 10.4103/pajo.pajo_36_24
5. Musk’s Neuralink gets FDA’s breakthrough device tag for ‘Blindsight’ implant, Reuters, 18 Sept 2024
6. World First: Neuralink Patient Makes YouTube Video With Brain Implant, 06 May 2025, Sciencealert.com, available from https://www.sciencealert.com/world-first-neuralink-patient-makes-youtube-video-with-brain-implant

